Effect of Oxidative Damage on the Stability and Dimerization of Superoxide Dismutase 1
- PMID: 27074676
- PMCID: PMC4833831
- DOI: 10.1016/j.bpj.2016.02.037
Effect of Oxidative Damage on the Stability and Dimerization of Superoxide Dismutase 1
Abstract
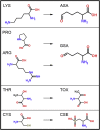
During their life cycle, proteins are subject to different modifications involving reactive oxygen species. Such oxidative damage to proteins may lead to the formation of insoluble aggregates and cytotoxicity and is associated with age-related disorders including neurodegenerative diseases, cancer, and diabetes. Superoxide dismutase 1 (SOD1), a key antioxidant enzyme in human cells, is particularly susceptible to such modifications. Moreover, this homodimeric metalloenzyme has been directly linked to both familial and sporadic amyotrophic lateral sclerosis (ALS), a devastating, late-onset motor neuronal disease, with more than 150 ALS-related mutations in the SOD1 gene. Importantly, oxidatively damaged SOD1 aggregates have been observed in both familial and sporadic forms of the disease. However, the molecular mechanisms as well as potential implications of oxidative stress in SOD1-induced cytotoxicity remain elusive. In this study, we examine the effects of oxidative modification on SOD1 monomer and homodimer stability, the key molecular properties related to SOD1 aggregation. We use molecular dynamics simulations in combination with thermodynamic integration to study microscopic-level site-specific effects of oxidative "mutations" at the dimer interface, including lysine, arginine, proline and threonine carbonylation, and cysteine oxidation. Our results show that oxidative damage of even single residues at the interface may drastically destabilize the SOD1 homodimer, with several modifications exhibiting a comparable effect to that of the most drastic ALS-causing mutations known. Additionally, we show that the SOD1 monomer stability decreases upon oxidative stress, which may lead to partial local unfolding and consequently to increased aggregation propensity. Importantly, these results suggest that oxidative stress may play a key role in development of ALS, with the mutations in the SOD1 gene being an additional factor.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Levine R.L., Stadtman E.R. Oxidative modification of proteins during aging. Exp. Gerontol. 2001;36:1495–1502. - PubMed
-
- Dalle-Donne I., Rossi R., Milzani A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006;52:601–623. - PubMed
-
- Davies K.J. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. - PubMed
-
- Grune T., Jung T., Davies K.J. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int. J. Biochem. Cell Biol. 2004;36:2519–2530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

