Automated Chemotactic Sorting and Single-cell Cultivation of Microbes using Droplet Microfluidics
- PMID: 27074762
- PMCID: PMC4831006
- DOI: 10.1038/srep24192
Automated Chemotactic Sorting and Single-cell Cultivation of Microbes using Droplet Microfluidics
Abstract
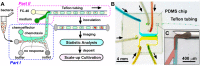
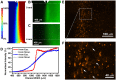
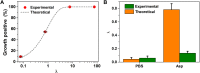
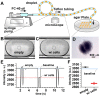
We report a microfluidic device for automated sorting and cultivation of chemotactic microbes from pure cultures or mixtures. The device consists of two parts: in the first part, a concentration gradient of the chemoeffector was built across the channel for inducing chemotaxis of motile cells; in the second part, chemotactic cells from the sample were separated, and mixed with culture media to form nanoliter droplets for encapsulation, cultivation, enumeration, and recovery of single cells. Chemotactic responses were assessed by imaging and statistical analysis of droplets based on Poisson distribution. An automated procedure was developed for rapid enumeration of droplets with cell growth, following with scale-up cultivation on agar plates. The performance of the device was evaluated by the chemotaxis assays of Escherichia coli (E. coli) RP437 and E. coli RP1616. Moreover, enrichment and isolation of non-labelled Comamonas testosteroni CNB-1 from its 1:10 mixture with E. coli RP437 was demonstrated. The enrichment factor reached 36.7 for CNB-1, based on its distinctive chemotaxis toward 4-hydroxybenzoic acid. We believe that this device can be widely used in chemotaxis studies without necessarily relying on fluorescent labelling, and isolation of functional microbial species from various environments.
Figures

References
-
- Berg H. C. Motile behavior of bacteria. Phys. Today. 53, 24–29 (2000).
-
- DiLuzio W. R. et al. Escherichia coli swim on the right-hand side. Nature. 435, 1271–1274 (2005). - PubMed
-
- Ottemann K. M. & Miller J. F. Roles for motility in bacterial-host interactions. Mol. Microbiol. 24, 1109–1117 (1997). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

