Proceedings of the 2015 National Toxicology Program Satellite Symposium
- PMID: 27075180
- PMCID: PMC5500208
- DOI: 10.1177/0192623316631844
Proceedings of the 2015 National Toxicology Program Satellite Symposium
Abstract
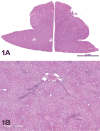
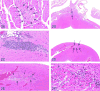
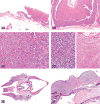
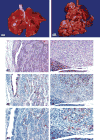
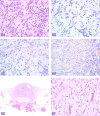
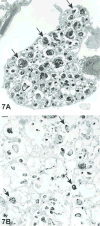
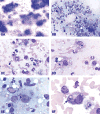
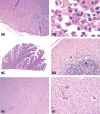
The 2015 Annual National Toxicology Program Satellite Symposium, entitled "Pathology Potpourri" was held in Minneapolis, Minnesota, at the American College of Veterinary Pathologists/American Society for Veterinary Clinical Pathology/Society of Toxicologic Pathology combined meeting. The goal of this symposium is to present and discuss diagnostic pathology challenges or nomenclature issues. Because of the combined meeting, both laboratory and domestic animal cases were presented. This article presents summaries of the speakers' talks, including challenging diagnostic cases or nomenclature issues that were presented, along with select images that were used for audience voting and discussion. Some lesions and topics covered during the symposium included hepatocellular lesions, a proposed harmonized diagnostic approach to rat cardiomyopathy, crop milk in a bird, avian feeding accoutrement, heat exchanger in a tuna, metastasis of a tobacco carcinogen-induced pulmonary carcinoma, neurocytoma in a rat, pituicytoma in a rat, rodent mammary gland whole mounts, dog and rat alveolar macrophage ultrastructure, dog and rat pulmonary phospholipidosis, alveolar macrophage aggregation in a dog, degenerating yeast in a cat liver aspirate, myeloid leukemia in lymph node aspirates from a dog, Trypanosoma cruzi in a dog, solanum toxicity in a cow, bovine astrovirus, malignant microglial tumor, and nomenclature challenges from the Special Senses International Harmonization of Nomenclature and Diagnostic Criteria Organ Working Group.
Keywords: INHAND; NTP Satellite Symposium; cardiomyopathy; crop milk; diagnostic neuropathology; focal nodular hyperplasia; histoplasmosis; leukemia +/− cytology; mammary gland whole mounts; neurocytoma; ocular inflammation; oral ornamentation; persistent fetal vasculature; persistent hyperplastic primary vitreous; persistent hyperplastic tunica vasculosa lentis; pulmonary metastatic tumor; pulmonary ultrastructure; tuna heat exchanger.
© The Author(s) 2016.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures










References
-
- Abcam product description website. Anti-CD68 antibody [ED1] (ab31630) Retrieved November 22, 2015: http://www.abcam.com/cd68-antibody-ed1-ab31630.html.
-
- Adams ET, Auerbach S, Blackshear PE, Bradley A, Gruebbel MM, Little PB, Malarkey D, Maronpot R, McKay JS, Miller RA, Moore RR, Morrison JP, Nyska A, Ramot Y, Rao D, Suttie A, Wells MY, Willson GA, Elmore SA. Proceedings of the 2010 National Toxicology Program Satellite Symposium. Toxicol Pathol. 2011;39:240–66. - PMC - PubMed
-
- Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004;444:527–35. - PubMed
-
- Allgoewer I, Pfefferkorn B. Persistent hyperplastic tunica vasculosa lentis and persistent hyperplastic primary vitreous (PHTVL/PHPV) in two cats. Vet Ophthalmol. 2001;4:161–4. - PubMed
-
- American Cancer Society. Breast Cancer Overview [Internet] Atlanta: American Cancer Society; c2014–15. [updated 2015 June 10; cited 2015 Nov 12]. Available at: http://www.cancer.org/Cancer/BreastCancer/OverviewGuide/breast-cancer-ov....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

