Libertas: a phase II placebo-controlled study of NRL001 in patients with faecal incontinence showed an unexpected and sustained placebo response
- PMID: 27075314
- PMCID: PMC4867152
- DOI: 10.1007/s00384-016-2585-7
Libertas: a phase II placebo-controlled study of NRL001 in patients with faecal incontinence showed an unexpected and sustained placebo response
Abstract
Purpose: Faecal incontinence (FI) is distressing, significantly reduces quality of life (QoL) and has few pharmacological treatments. The α1-adrenoceptor agonist NRL001 (1R,2S-methoxamine hydrochloride) improves anal sphincter tone. NRL001 efficacy was evaluated by changes in Wexner scores at week 4 vs. baseline in NRL001-treated patients compared with placebo. Impact of NRL001 on QoL and safety were also assessed.
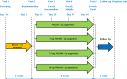
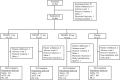
Methods: Four hundred sixty-six patients received NRL001 (5, 7.5 or 10 mg) or placebo as suppository, once daily over 8 weeks. Wexner score, Vaizey score and QoL were analysed at baseline, week 4 and week 8. FI episodes and adverse events were recorded in diaries.
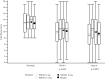
Results: At week 4, mean reductions in Wexner scores were -3.0, -2.6, -2.6 and -2.4 for NRL001 5, 7.5, 10 mg and placebo, respectively. All reduced further by week 8. As placebo responses also improved, there was no significant treatment effect at week 4 (p = 0.6867) or week 8 (p = 0.5005). FI episode frequency improved for all patients, but not significantly compared with placebo (week 4: p = 0.2619, week 8: p = 0.5278). All patients' QoL improved, but not significantly for all parameters (p > 0.05) except depression/self-perception at week 4 (p = 0.0102) and week 8 (p = 0.0069), compared with placebo. Most adverse events were mild and judged probably or possibly related to NRL001.
Conclusions: All groups demonstrated improvement in efficacy and QoL compared with baseline. NRL001 was well-tolerated without serious safety concerns. Despite the improvement in all groups, there was no statistically significant treatment effect, underlining the importance of relating results to a placebo arm.
Keywords: Alpha-1 receptor agonist; Episode frequency; Faecal incontinence; NRL001; Quality of life.
Figures

Similar articles
-
Libertas: rationale and study design of a multicentre, Phase II, double-blind, randomised, placebo-controlled investigation to evaluate the efficacy, safety and tolerability of locally applied NRL001 in patients with faecal incontinence.Colorectal Dis. 2014 Mar;16 Suppl 1:59-66. doi: 10.1111/codi.12546. Colorectal Dis. 2014. PMID: 24499497 Clinical Trial.
-
A double-blind, placebo-controlled, randomised, parallel-group, dose-escalating, repeat dose study in healthy volunteers to evaluate the safety, tolerability, pharmacodynamic effects and pharmacokinetics of the once daily rectal application of NRL001 suppositories for 14 days.Colorectal Dis. 2014 Mar;16 Suppl 1:36-50. doi: 10.1111/codi.12544. Colorectal Dis. 2014. PMID: 24499495 Clinical Trial.
-
A randomised, controlled, crossover study to investigate the safety and response of 1R,2S-methoxamine hydrochloride (NRL001) on anal function in healthy volunteers.Colorectal Dis. 2014 Mar;16 Suppl 1:5-15. doi: 10.1111/codi.12541. Colorectal Dis. 2014. PMID: 24499492 Clinical Trial.
-
Clinical effectiveness and safety of self-expandable implantable bulking agents for faecal incontinence: a systematic review.BMC Gastroenterol. 2022 Aug 17;22(1):389. doi: 10.1186/s12876-022-02441-4. BMC Gastroenterol. 2022. PMID: 35978293 Free PMC article.
-
Darifenacin, an M3 selective receptor antagonist, is an effective and well-tolerated once-daily treatment for overactive bladder.Eur Urol. 2004 Apr;45(4):420-9; discussion 429. doi: 10.1016/j.eururo.2004.01.008. Eur Urol. 2004. PMID: 15041104 Review.
Cited by
-
Tools for fecal incontinence assessment: lessons for inflammatory bowel disease trials based on a systematic review.United European Gastroenterol J. 2020 Oct;8(8):886-922. doi: 10.1177/2050640620943699. Epub 2020 Jul 17. United European Gastroenterol J. 2020. PMID: 32677555 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

