Immunogenicity of Leishmania-derived hepatitis B small surface antigen particles exposing highly conserved E2 epitope of hepatitis C virus
- PMID: 27075377
- PMCID: PMC4831159
- DOI: 10.1186/s12934-016-0460-4
Immunogenicity of Leishmania-derived hepatitis B small surface antigen particles exposing highly conserved E2 epitope of hepatitis C virus
Abstract
Background: Hepatitis C virus (HCV) infection is a major health problem worldwide, affecting an estimated 2-3 % of human population. An HCV vaccine, however, remains unavailable. High viral diversity poses a challenge in developing a vaccine capable of eliciting a broad neutralizing antibody response against all HCV genotypes. The small surface antigen (sHBsAg) of hepatitis B virus (HBV) has the ability to form highly immunogenic subviral particles which are currently used as an efficient anti-HBV vaccine. It also represents an attractive antigen carrier for the delivery of foreign sequences. In the present study, we propose a bivalent vaccine candidate based on novel chimeric particles in which highly conserved epitope of HCV E2 glycoprotein (residues 412-425) was inserted into the hydrophilic loop of sHBsAg.
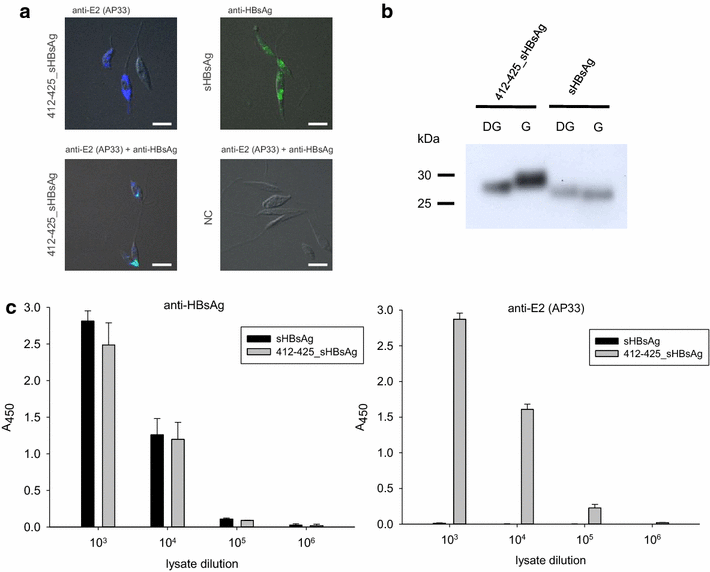
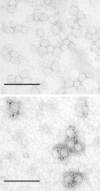
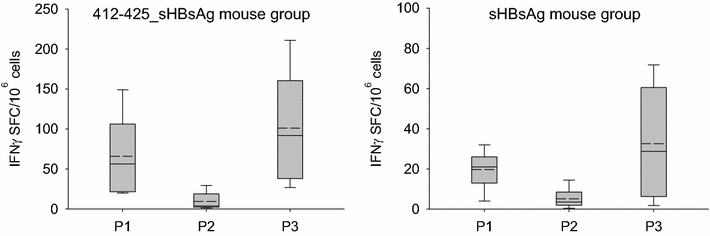
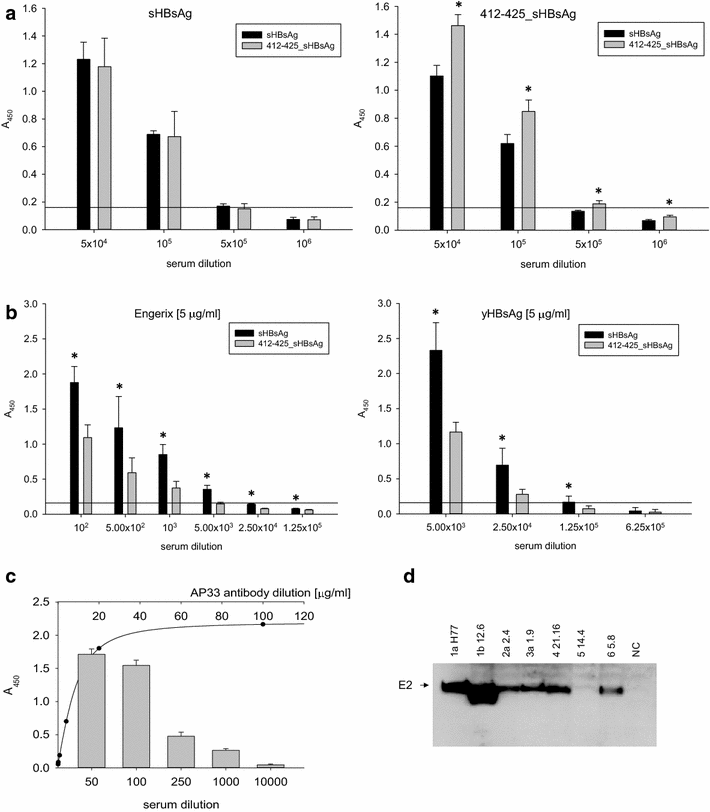
Results: The expression of chimeric protein was performed in an unconventional, Leishmania tarentolae expression system resulting in an assembly of particles which retained immunogenicity of both HCV epitope and sHBsAg protein. Direct transmission electron microscopy observation and immunogold staining confirmed the formation of spherical particles approximately 22 nm in diameter, and proper foreign epitope exposition. Furthermore, the sera of mice immunized with chimeric particles proved reactive not only to purified yeast-derived sHBsAg proteins but also HCV E2 412-425 synthetic peptide. Most importantly, they were also able to cross-react with E1E2 complexes from different HCV genotypes.
Conclusions: For the first time, we confirmed successful assembly of chimeric sHBsAg virus-like particles (VLPs) in the L. tarentolae expression system which has the potential to produce high-yields of properly N-glycosylated mammalian proteins. We also proved that chimeric Leishmania-derived VLPs are highly immunogenic and able to elicit cross-reactive antibody response against HCV. This approach may prove useful in the development of a bivalent prophylactic vaccine against HBV and HCV and opens up a new and low-cost opportunity for the production of chimeric sHBsAg VLPs requiring N-glycosylation process for their proper functionality and immunogenicity.
Keywords: HBV small surface antigen (sHBsAg); Hepatitis C virus (HCV); Leishmania tarentolae; VLP; Vaccine.
Figures
Similar articles
-
Minicircle-based vaccine induces potent T-cell and antibody responses against hepatitis C virus.Sci Rep. 2024 Nov 4;14(1):26698. doi: 10.1038/s41598-024-78049-3. Sci Rep. 2024. PMID: 39496832 Free PMC article.
-
Neutralization effects of antibody elicited by chimeric HBV S antigen viral-like particles presenting HCV neutralization epitopes.Vaccine. 2018 Apr 19;36(17):2273-2281. doi: 10.1016/j.vaccine.2018.03.036. Epub 2018 Mar 22. Vaccine. 2018. PMID: 29576303
-
Effect of Glycan Shift on Antibodies against Hepatitis C Virus E2 412-425 Epitope Elicited by Chimeric sHBsAg-Based Virus-Like Particles.Microbiol Spectr. 2023 Jan 31;11(2):e0254622. doi: 10.1128/spectrum.02546-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36719195 Free PMC article.
-
Hybrid hepatitis B virus core antigen as a vaccine carrier moiety: I. presentation of foreign epitopes.J Biotechnol. 1996 Jan 26;44(1-3):91-6. doi: 10.1016/0168-1656(95)00118-2. J Biotechnol. 1996. PMID: 8717391 Review.
-
Hepatitis C Virus E1E2 Structure, Diversity, and Implications for Vaccine Development.Viruses. 2024 May 18;16(5):803. doi: 10.3390/v16050803. Viruses. 2024. PMID: 38793684 Free PMC article. Review.
Cited by
-
Leishmania tarentolae as a platform for the production of vaccines against viral pathogens.NPJ Vaccines. 2024 Nov 6;9(1):212. doi: 10.1038/s41541-024-01005-9. NPJ Vaccines. 2024. PMID: 39505865 Free PMC article. Review.
-
Immunization with Leishmania tarentolae-derived norovirus virus-like particles elicits high humoral response and stimulates the production of neutralizing antibodies.Microb Cell Fact. 2021 Sep 24;20(1):186. doi: 10.1186/s12934-021-01677-1. Microb Cell Fact. 2021. PMID: 34560881 Free PMC article.
-
Chimeric virus-like particles presenting tumour-associated MUC1 epitope result in high titers of specific IgG antibodies in the presence of squalene oil-in-water adjuvant: towards safe cancer immunotherapy.J Nanobiotechnology. 2022 Mar 27;20(1):160. doi: 10.1186/s12951-022-01357-1. J Nanobiotechnology. 2022. PMID: 35351156 Free PMC article.
-
Structural and Epitope Analysis (T- and B-Cell Epitopes) of Hepatitis C Virus (HCV) Glycoproteins: An in silico Approach.J Clin Exp Hepatol. 2018 Dec;8(4):352-361. doi: 10.1016/j.jceh.2017.12.010. Epub 2018 Feb 21. J Clin Exp Hepatol. 2018. PMID: 30568344 Free PMC article.
-
Application of built-in adjuvants for epitope-based vaccines.PeerJ. 2019 Jan 14;6:e6185. doi: 10.7717/peerj.6185. eCollection 2019. PeerJ. 2019. PMID: 30656066 Free PMC article.
References
-
- ION-1 Investigators Ledipasvir and Sofosbuvir for Untreated HCV Genotype 1 Infection. N Engl J Med. 2014 - PubMed
-
- Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

