Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms
- PMID: 27075392
- PMCID: PMC4834629
- DOI: 10.1038/ncomms11220
Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms
Abstract
Many bacteria produce extracellular and surface-associated components such as membrane vesicles (MVs), extracellular DNA and moonlighting cytosolic proteins for which the biogenesis and export pathways are not fully understood. Here we show that the explosive cell lysis of a sub-population of cells accounts for the liberation of cytosolic content in Pseudomonas aeruginosa biofilms. Super-resolution microscopy reveals that explosive cell lysis also produces shattered membrane fragments that rapidly form MVs. A prophage endolysin encoded within the R- and F-pyocin gene cluster is essential for explosive cell lysis. Endolysin-deficient mutants are defective in MV production and biofilm development, consistent with a crucial role in the biogenesis of MVs and liberation of extracellular DNA and other biofilm matrix components. Our findings reveal that explosive cell lysis, mediated through the activity of a cryptic prophage endolysin, acts as a mechanism for the production of bacterial MVs.
Figures
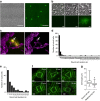
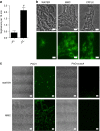

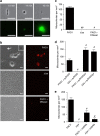

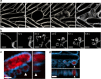

Comment in
-
Biofilms: Have exploding cells blown up MV dogma?Nat Rev Microbiol. 2016 Jun;14(6):334-5. doi: 10.1038/nrmicro.2016.68. Epub 2016 Apr 25. Nat Rev Microbiol. 2016. PMID: 27108707 No abstract available.
References
-
- Kaparakis-Liaskos M. & Ferrero R. L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 15, 375–387 (2015). - PubMed
-
- Toyofuku M., Roschitzki B., Riedel K. & Eberl L. Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. J. Proteome Res. 11, 4906–4915 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

