DRAMP: a comprehensive data repository of antimicrobial peptides
- PMID: 27075512
- PMCID: PMC4830929
- DOI: 10.1038/srep24482
DRAMP: a comprehensive data repository of antimicrobial peptides
Abstract
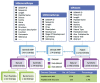
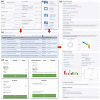
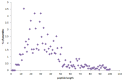
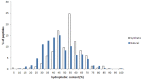
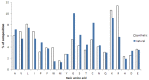
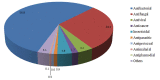
The growing problem of antibiotic-resistant microorganisms results in an urgent need for substitutes to conventional antibiotics with novel modes of action and effective activities. Antimicrobial peptides (AMPs), produced by a wide variety of living organisms acting as a defense mechanism against invading pathogenic microbes, are considered to be such promising alternatives. AMPs display a broad spectrum of antimicrobial activity and a low propensity for developing resistance. Therefore, a thorough understanding of AMPs is essential to exploit them as antimicrobial drugs. Considering this, we developed a comprehensive user-friendly data repository of antimicrobial peptides (DRAMP), which holds 17349 antimicrobial sequences, including 4571 general AMPs, 12704 patented sequences and 74 peptides in drug development. Entries in the database have detailed annotations, especially detailed antimicrobial activity data (shown as target organism with MIC value) and structure information. Annotations also include accession numbers crosslinking to Pubmed, Swiss-prot and Protein Data Bank (PDB). The website of the database comes with easy-to-operate browsing as well as searching with sorting and filtering functionalities. Several useful sequence analysis tools are provided, including similarity search, sequence alignment and conserved domain search (CD-Search). DRAMP should be a useful resource for the development of novel antimicrobial peptide drugs.
Figures
Similar articles
-
DRAMP 2.0, an updated data repository of antimicrobial peptides.Sci Data. 2019 Aug 13;6(1):148. doi: 10.1038/s41597-019-0154-y. Sci Data. 2019. PMID: 31409791 Free PMC article.
-
dbAMP: an integrated resource for exploring antimicrobial peptides with functional activities and physicochemical properties on transcriptome and proteome data.Nucleic Acids Res. 2019 Jan 8;47(D1):D285-D297. doi: 10.1093/nar/gky1030. Nucleic Acids Res. 2019. PMID: 30380085 Free PMC article.
-
DRAMP 3.0: an enhanced comprehensive data repository of antimicrobial peptides.Nucleic Acids Res. 2022 Jan 7;50(D1):D488-D496. doi: 10.1093/nar/gkab651. Nucleic Acids Res. 2022. PMID: 34390348 Free PMC article.
-
The expanding scope of antimicrobial peptide structures and their modes of action.Trends Biotechnol. 2011 Sep;29(9):464-72. doi: 10.1016/j.tibtech.2011.05.001. Epub 2011 Jun 15. Trends Biotechnol. 2011. PMID: 21680034 Review.
-
Antimicrobial Peptides: A Promising Avenue for Human Healthcare.Curr Pharm Biotechnol. 2020;21(2):90-96. doi: 10.2174/1389201020666191011121722. Curr Pharm Biotechnol. 2020. PMID: 31612826 Review.
Cited by
-
Antimicrobial peptides - Advances in development of therapeutic applications.Life Sci. 2020 Nov 1;260:118407. doi: 10.1016/j.lfs.2020.118407. Epub 2020 Sep 12. Life Sci. 2020. PMID: 32931796 Free PMC article. Review.
-
MLAFP-XN: Leveraging neural network model for development of antifungal peptide identification tool.Heliyon. 2024 Sep 11;10(18):e37820. doi: 10.1016/j.heliyon.2024.e37820. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39323787 Free PMC article.
-
A D-enantiomer of the antimicrobial peptide GL13K evades antimicrobial resistance in the Gram positive bacteria Enterococcus faecalis and Streptococcus gordonii.PLoS One. 2018 Mar 22;13(3):e0194900. doi: 10.1371/journal.pone.0194900. eCollection 2018. PLoS One. 2018. PMID: 29566082 Free PMC article.
-
Antibiofilm Peptides and Peptidomimetics with Focus on Surface Immobilization.Biomolecules. 2018 May 16;8(2):27. doi: 10.3390/biom8020027. Biomolecules. 2018. PMID: 29772735 Free PMC article. Review.
-
Antimicrobial peptide CGA-N12 decreases the Candida tropicalis mitochondrial membrane potential via mitochondrial permeability transition pore.Biosci Rep. 2020 May 29;40(5):BSR20201007. doi: 10.1042/BSR20201007. Biosci Rep. 2020. PMID: 32368781 Free PMC article.
References
-
- Cruz J., Ortiz C., Guzman F., Fernandez-Lafuente R. & Torres R. Antimicrobial peptides: promising compounds against pathogenic microorganisms. Curr. Med. Chem. 21, 2299–2321 (2014). - PubMed
-
- Li Y., Xiang Q., Zhang Q., Huang Y. & Su Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 37, 207–215 (2012). - PubMed
-
- Giuliani A., Pirri G. & Nicoletto S. F. Antimicrobial peptides: an overview of a promising class of therapeutics. Cent. Eur. J. Biol. 2, 1–33 (2007).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

