Structural and functional properties of prefibrillar α-synuclein oligomers
- PMID: 27075649
- PMCID: PMC4830946
- DOI: 10.1038/srep24526
Structural and functional properties of prefibrillar α-synuclein oligomers
Abstract
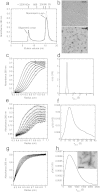
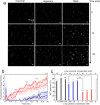
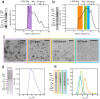
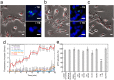
The deposition of fibrillar alpha-synuclein (α-syn) within inclusions (Lewy bodies and Lewy neurites) in neurons and glial cells is a hallmark of synucleinopathies. α-syn populates a variety of assemblies ranging from prefibrillar oligomeric species to fibrils whose specific contribution to neurodegeneration is still unclear. Here, we compare the specific structural and biological properties of distinct soluble prefibrillar α-syn oligomers formed either spontaneously or in the presence of dopamine and glutaraldehyde. We show that both on-fibrillar assembly pathway and distinct dopamine-mediated and glutaraldehyde-cross-linked α-syn oligomers are only slightly effective in perturbing cell membrane integrity and inducing cytotoxicity, while mature fibrils exhibit the highest toxicity. In contrast to low-molecular weight and unstable oligomers, large stable α-syn oligomers seed the aggregation of soluble α-syn within reporter cells although to a lesser extent than mature α-syn fibrils. These oligomers appear elongated in shape. Our findings suggest that α-syn oligomers represent a continuum of species ranging from unstable low molecular weight particles to mature fibrils via stable elongated oligomers composed of more than 15 α-syn monomers that possess seeding capacity.
Figures

Similar articles
-
Polymorphic α-Synuclein Strains Modified by Dopamine and Docosahexaenoic Acid Interact Differentially with Tau Protein.Mol Neurobiol. 2020 Jun;57(6):2741-2765. doi: 10.1007/s12035-020-01913-6. Epub 2020 Apr 29. Mol Neurobiol. 2020. PMID: 32350746 Free PMC article.
-
Fibrillar α-synuclein and huntingtin exon 1 assemblies are toxic to the cells.Biophys J. 2012 Jun 20;102(12):2894-905. doi: 10.1016/j.bpj.2012.04.050. Epub 2012 Jun 19. Biophys J. 2012. PMID: 22735540 Free PMC article.
-
α-Synuclein strains cause distinct synucleinopathies after local and systemic administration.Nature. 2015 Jun 18;522(7556):340-4. doi: 10.1038/nature14547. Epub 2015 Jun 10. Nature. 2015. PMID: 26061766
-
α-synuclein oligomers and fibrils: a spectrum of species, a spectrum of toxicities.J Neurochem. 2019 Sep;150(5):522-534. doi: 10.1111/jnc.14808. Epub 2019 Aug 2. J Neurochem. 2019. PMID: 31254394 Review.
-
Phase separation and other forms of α-Synuclein self-assemblies.Essays Biochem. 2022 Dec 16;66(7):987-1000. doi: 10.1042/EBC20220055. Essays Biochem. 2022. PMID: 36373662 Review.
Cited by
-
Lipids uniquely alter rates of insulin aggregation and lower toxicity of amyloid aggregates.Biochim Biophys Acta Mol Cell Biol Lipids. 2023 Jan;1868(1):159247. doi: 10.1016/j.bbalip.2022.159247. Epub 2022 Oct 20. Biochim Biophys Acta Mol Cell Biol Lipids. 2023. PMID: 36272517 Free PMC article.
-
Understanding the Multiple Role of Mitochondria in Parkinson's Disease and Related Disorders: Lesson From Genetics and Protein-Interaction Network.Front Cell Dev Biol. 2021 Apr 1;9:636506. doi: 10.3389/fcell.2021.636506. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869180 Free PMC article. Review.
-
Nanoscale Characterization of Parallel and Antiparallel β-Sheet Amyloid Beta 1-42 Aggregates.ACS Chem Neurosci. 2022 Oct 5;13(19):2813-2820. doi: 10.1021/acschemneuro.2c00180. Epub 2022 Sep 19. ACS Chem Neurosci. 2022. PMID: 36122250 Free PMC article.
-
In Search of Effective Treatments Targeting α-Synuclein Toxicity in Synucleinopathies: Pros and Cons.Front Cell Dev Biol. 2020 Sep 4;8:559791. doi: 10.3389/fcell.2020.559791. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015057 Free PMC article. Review.
-
The Overcrowded Crossroads: Mitochondria, Alpha-Synuclein, and the Endo-Lysosomal System Interaction in Parkinson's Disease.Int J Mol Sci. 2019 Oct 25;20(21):5312. doi: 10.3390/ijms20215312. Int J Mol Sci. 2019. PMID: 31731450 Free PMC article. Review.
References
-
- McCann H., Stevens C. H., Cartwright H. & Halliday G. M. α-Synucleinopathy phenotypes. Parkinsonism Relat. Disord. 20, S62–67 (2014). - PubMed
-
- Spillantini M. G. et al.. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 251, 205–208 (1998). - PubMed
-
- Crowther R. A., Jakes R., Spillantini M. G. & Goedert M. Synthetic filaments assembled from C-terminally truncated alpha-synuclein. FEBS Lett. 436, 309–312 (1998). - PubMed
-
- Lashuel H. A. et al.. Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J. Mol. Biol. 322, 1089–1102 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

