miR-141-Mediated Regulation of Brain Metastasis From Breast Cancer
- PMID: 27075851
- PMCID: PMC5017951
- DOI: 10.1093/jnci/djw026
miR-141-Mediated Regulation of Brain Metastasis From Breast Cancer
Abstract
Background: Brain metastasis poses a major treatment challenge and remains an unmet clinical need. Finding novel therapies to prevent and treat brain metastases requires an understanding of the biology and molecular basis of the process, which currently is constrained by a dearth of experimental models and specific therapeutic targets.
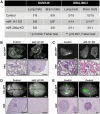
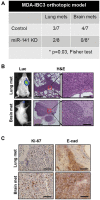
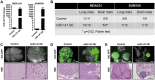
Methods: Green Fluorescent Protein (GFP)-labeled breast cancer cells were injected via tail vein into SCID/Beige mice (n = 10-15 per group), and metastatic colonization to the brain and lung was evaluated eight weeks later. Knockdown and overexpression of miR-141 were achieved with lentiviral vectors. Serum levels of miR-141 were measured from breast cancer patients (n = 105), and the association with clinical outcome was determined by Kaplan-Meier method. All statistical tests were two-sided.
Results: Novel brain metastasis mouse models were developed via tail vein injection of parental triple-negative and human epidermal growth factor receptor 2 (HER2)-overexpressing inflammatory breast cancer lines. Knockdown of miR-141 inhibited metastatic colonization to brain (miR-141 knockdown vs control: SUM149, 0/8 mice vs 6/9 mice,P= .009; MDA-IBC3, 2/14 mice vs 10/15 mice,P= .007). Ectopic expression of miR-141 in nonexpressing MDA-MB-231 enhanced brain metastatic colonization (5/9 mice vs 0/10 mice,P= .02). Furthermore, high miR-141 serum levels were associated with shorter brain metastasis-free survival (P= .04) and were an independent predictor of progression-free survival (hazard ratio [HR] = 4.77, 95% confidence interval [CI] = 2.61 to 8.71,P< .001) and overall survival (HR = 7.22, 95% CI = 3.46 to 15.06,P< .001).
Conclusions: Our study suggests miR-141 is a regulator of brain metastasis from breast cancer and should be examined as a biomarker and potential target to prevent and treat brain metastases.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures





References
-
- Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. - PubMed
-
- Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13(6):1648–1655. - PubMed
-
- Langley RR, Fidler IJ. The biology of brain metastasis. Clin Chem. 2013;59(1):180–189. - PubMed
-
- Fidler IJ. The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011;21(2):107–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

