Targeting a Cancer-Specific Epitope of the Epidermal Growth Factor Receptor in Triple-Negative Breast Cancer
- PMID: 27075852
- PMCID: PMC5017938
- DOI: 10.1093/jnci/djw028
Targeting a Cancer-Specific Epitope of the Epidermal Growth Factor Receptor in Triple-Negative Breast Cancer
Abstract
Background: Triple-negative breast cancers (TNBCs) are typically more aggressive and result in poorer outcomes than other breast cancers because treatment options are limited due to lack of hormone receptors or amplified human epidermal growth factor receptor 2 (HER2). Many TNBCs overexpress the epidermal growth factor receptor (EGFR) or manifest amplification of theEGFRgene, supporting EGFR as a therapeutic target. While EGFR-directed small molecule inhibitors have shown limited effectiveness in clinical settings, use of EGFR as a mechanism of delivering enzymatic cytotoxins to TNBC has not been demonstrated.
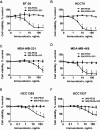
Methods: Using the single-chain variable fragment (scFv) of the 806 antibody that binds only cells with overexpressed, misfolded, or mutant variants of the EGFR, a recombinant immunotoxin was engineered through gene fusion withPseudomonas aeruginosaExotoxin A (806-PE38). The potency of 806-PE38 on reducing TNBC cell growth in vitro and in xenograft models (n ≥ 6) was examined for six TNBC cell lines. All statistical tests were two-sided.
Results: 806-PE38 statistically significantly reduced the viability of all tested TNBC lines, with IC50values below 10 ng/mL for three of six cell lines, while not affecting cells with wild-type EGFR (IC50>300 ng/mL). Systemic treatments with 806-PE38 vs vehicle resulted in statistically significantly reduced tumor burdens (806-PE38 mean = 128 mm(3)[SD = 46 mm(3)] vs vehicle mean = 749 mm(3)[SD = 395 mm(3)], P = .001) and increased median survival (806-PE38 median = 82 days vs vehicle median = 50 days,P= .01) in a MDA-MB-468 TNBC mouse xenograft. Deletion of the catalytic residue eliminated both cytotoxic activity in vitro and the reduction in tumor burden and survival (P= .52).
Conclusions: These data support the further development of the 806-PE38 immunotoxin as a therapeutic agent for the treatment of patients with EGFR-positive TNBC. Follow-up experiments with combination therapies will be attempted to achieve full remissions.
Published by Oxford University Press 2016. This work is written by US Government employees and is in the public domain in the United States.
Figures



Similar articles
-
Engineered Anti-GPC3 Immunotoxin, HN3-ABD-T20, Produces Regression in Mouse Liver Cancer Xenografts Through Prolonged Serum Retention.Hepatology. 2020 May;71(5):1696-1711. doi: 10.1002/hep.30949. Epub 2020 Jan 27. Hepatology. 2020. PMID: 31520528 Free PMC article.
-
In vitro and in vivo cytotoxic activities of recombinant immunotoxin 8H9(Fv)-PE38 against breast cancer, osteosarcoma, and neuroblastoma.Cancer Res. 2004 Feb 15;64(4):1419-24. doi: 10.1158/0008-5472.can-03-0570. Cancer Res. 2004. PMID: 14973056
-
HER2-targeted immunotoxins with low nonspecific toxicity and immunogenicity.Biochem Biophys Res Commun. 2016 Jun 17;475(1):93-9. doi: 10.1016/j.bbrc.2016.05.044. Epub 2016 May 10. Biochem Biophys Res Commun. 2016. PMID: 27178207
-
Cintredekin besudotox in treatment of malignant glioma.Expert Opin Biol Ther. 2008 Jun;8(6):805-12. doi: 10.1517/14712598.8.6.805. Expert Opin Biol Ther. 2008. PMID: 18476792 Review.
-
Targeted toxins in brain tumor therapy.Toxins (Basel). 2010 Nov;2(11):2645-62. doi: 10.3390/toxins2112645. Epub 2010 Nov 1. Toxins (Basel). 2010. PMID: 22069569 Free PMC article. Review.
Cited by
-
Triple negative breast cancer: shedding light onto the role of pi3k/akt/mtor pathway.Oncotarget. 2016 Sep 13;7(37):60712-60722. doi: 10.18632/oncotarget.10858. Oncotarget. 2016. PMID: 27474173 Free PMC article. Review.
-
Targeting dePARylation selectively suppresses DNA repair-defective and PARP inhibitor-resistant malignancies.Sci Adv. 2019 Apr 10;5(4):eaav4340. doi: 10.1126/sciadv.aav4340. eCollection 2019 Apr. Sci Adv. 2019. PMID: 30989114 Free PMC article.
-
Immunotoxin Therapies for the Treatment of Epidermal Growth Factor Receptor-Dependent Cancers.Toxins (Basel). 2016 May 4;8(5):137. doi: 10.3390/toxins8050137. Toxins (Basel). 2016. PMID: 27153091 Free PMC article. Review.
-
Eugenol-Induced Autophagy and Apoptosis in Breast Cancer Cells via PI3K/AKT/FOXO3a Pathway Inhibition.Int J Mol Sci. 2021 Aug 26;22(17):9243. doi: 10.3390/ijms22179243. Int J Mol Sci. 2021. PMID: 34502165 Free PMC article.
-
Targeting Receptors on Cancer Cells with Protein Toxins.Biomolecules. 2020 Sep 17;10(9):1331. doi: 10.3390/biom10091331. Biomolecules. 2020. PMID: 32957689 Free PMC article. Review.
References
-
- Dent R, Trudeau M, Pritchard KI, et al. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin Cancer Res. 2007;13(15):4429–4434. - PubMed
-
- Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23:vi7–vi12. - PubMed
-
- Liedtke C, Mazouni C, Hess KR, et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. J Clin Oncol. 2008;26(8):1275–1281. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

