Tuning of AKT-pathway by Nef and its blockade by protease inhibitors results in limited recovery in latently HIV infected T-cell line
- PMID: 27076174
- PMCID: PMC4831010
- DOI: 10.1038/srep24090
Tuning of AKT-pathway by Nef and its blockade by protease inhibitors results in limited recovery in latently HIV infected T-cell line
Abstract
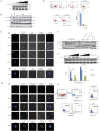
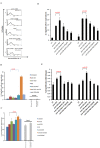
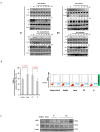
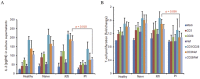
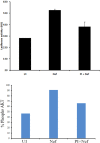
Akt signaling plays a central role in many biological processes, which are key players in human immunodeficiency virus 1 (HIV-1) pathogenesis. We found that Akt interacts with HIV-1 Nef protein. In primary T cells treated with exogenous Nef or acutely infected with Nef-expressing HIV-1 in vitro, Akt became phosphorylated on serine(473) and threonine(308). In vitro, Akt activation mediated by Nef in T-cells was blocked by HIV protease inhibitors (PI), but not by reverse transcriptase inhibitors (RTI). Ex vivo, we found that the Akt pathway is hyperactivated in peripheral blood lymphocytes (PBLs) from cART naïve HIV-1-infected patients. PBLs isolated from PI-treated patients, but not from RTI-treated patients, exhibited decreased Akt activation, T-cell proliferation and IL-2 production. We found that PI but not RTI can block HIV-1 reactivation in latently infected J-Lat lymphoid cells stimulated with various stimuli. Using luciferase measurement, we further confirmed that Nef-mediated reactivation of HIV-1 from latency in 1G5 cells was blocked by PI parallel to decreased Akt activation. Our results indicate that PI-mediated blockade of Akt activation could impact the HIV-1 reservoir and support the need to further assess the therapeutic use of HIV-1 PI in order to curtail latently infected cells in HIV-1-infected patients.
Conflict of interest statement
G.H. and O.R. are editorial board members. The authors declare no other conflict of interest.
Figures



References
-
- Fayard E., Xue G., Parcellier A., Bozulic L. & Hemmings B. A. Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Curr. Top. Microbiol. Immunol. 346, 31–56 (2010). - PubMed
-
- Malim M. H. & Emerman M. HIV-1 accessory proteins–ensuring viral survival in a hostile environment. Cell Host. Microbe 3, 388–398 (2008). - PubMed
-
- Maartens G., Celum C. & Lewin S. R. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet 384, 258–271 (2014). - PubMed
-
- Deng K. & Siliciano R. F. HIV: Early treatment may not be early enough. Nature 512, 35–36 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

