Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia
- PMID: 27076371
- PMCID: PMC5448406
- DOI: 10.1158/2159-8290.CD-16-0040
Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia
Abstract
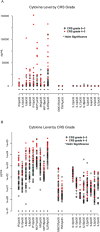
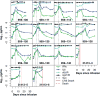
Chimeric antigen receptor (CAR)-modified T cells with anti-CD19 specificity are a highly effective novel immune therapy for relapsed/refractory acute lymphoblastic leukemia. Cytokine release syndrome (CRS) is the most significant and life-threatening toxicity. To improve understanding of CRS, we measured cytokines and clinical biomarkers in 51 CTL019-treated patients. Peak levels of 24 cytokines, including IFNγ, IL6, sgp130, and sIL6R, in the first month after infusion were highly associated with severe CRS. Using regression modeling, we could accurately predict which patients would develop severe CRS with a signature composed of three cytokines. Results were validated in an independent cohort. Changes in serum biochemical markers, including C-reactive protein and ferritin, were associated with CRS but failed to predict development of severe CRS. These comprehensive profiling data provide novel insights into CRS biology and, importantly, represent the first data that can accurately predict which patients have a high probability of becoming critically ill.
Significance: CRS is the most common severe toxicity seen after CAR T-cell treatment. We developed models that can accurately predict which patients are likely to develop severe CRS before they become critically ill, which improves understanding of CRS biology and may guide future cytokine-directed therapy. Cancer Discov; 6(6); 664-79. ©2016 AACR.See related commentary by Rouce and Heslop, p. 579This article is highlighted in the In This Issue feature, p. 561.
©2016 American Association for Cancer Research.
Conflict of interest statement
Figures


Comment in
-
Forecasting Cytokine Storms with New Predictive Biomarkers.Cancer Discov. 2016 Jun;6(6):579-80. doi: 10.1158/2159-8290.CD-16-0493. Cancer Discov. 2016. PMID: 27261481 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

