Endogenous Transmembrane TNF-Alpha Protects Against Premature Senescence in Endothelial Colony Forming Cells
- PMID: 27076598
- PMCID: PMC4867129
- DOI: 10.1161/CIRCRESAHA.116.308332
Endogenous Transmembrane TNF-Alpha Protects Against Premature Senescence in Endothelial Colony Forming Cells
Abstract
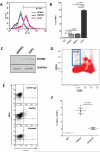
Rationale: Transmembrane tumor necrosis factor-α (tmTNF-α) is the prime ligand for TNF receptor 2, which has been shown to mediate angiogenic and blood vessel repair activities in mice. We have previously reported that the angiogenic potential of highly proliferative endothelial colony-forming cells (ECFCs) can be explained by the absence of senescent cells, which in mature endothelial cells occupy >30% of the population, and that exposure to a chronic inflammatory environment induced premature, telomere-independent senescence in ECFCs.
Objective: The goal of this study was to determine the role of tmTNF-α in the proliferation of ECFCs.
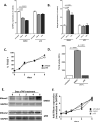
Methods and results: Here, we show that tmTNF-α expression on ECFCs selects for higher proliferative potential and when removed from the cell surface promotes ECFC senescence. Moreover, the induction of premature senescence by chronic inflammatory conditions is blocked by inhibition of tmTNF-α cleavage. Indeed, the mechanism of chronic inflammation-induced premature senescence involves an abrogation of tmTNF/TNF receptor 2 signaling. This process is mediated by activation of the tmTNF cleavage metalloprotease TNF-α-converting enzyme via p38 MAP kinase activation and its concurrent export to the cell surface by means of increased iRhom2 expression.
Conclusions: Thus, we conclude that tmTNF-α on the surface of highly proliferative ECFCs plays an important role in the regulation of their proliferative capacity.
Keywords: apoptosis; endothelium; inflammation; metalloprotease; vasodilation.
© 2016 American Heart Association, Inc.
Figures






References
-
- Wang YX, Fitch RM. Vascular stiffness: measurements, mechanisms and implications. Curr Vasc Pharmacol. 2004;2:379–84. - PubMed
-
- Op den Buijs J, Musters M, Verrips T, Post JA, Braam B, van Riel N. Mathematical modeling of vascular endothelial layer maintenance: the role of endothelial cell division, progenitor cell homing, and telomere shortening. Am J Physiol Heart Circ Physiol. 2004;287:H2651–8. - PubMed
-
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Issner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

