Modeling Barrier Tissues In Vitro: Methods, Achievements, and Challenges
- PMID: 27077109
- PMCID: PMC4816829
- DOI: 10.1016/j.ebiom.2016.02.023
Modeling Barrier Tissues In Vitro: Methods, Achievements, and Challenges
Abstract
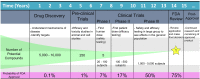
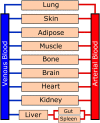
Organ-on-a-chip devices have gained attention in the field of in vitro modeling due to their superior ability in recapitulating tissue environments compared to traditional multiwell methods. These constructed growth environments support tissue differentiation and mimic tissue-tissue, tissue-liquid, and tissue-air interfaces in a variety of conditions. By closely simulating the in vivo biochemical and biomechanical environment, it is possible to study human physiology in an organ-specific context and create more accurate models of healthy and diseased tissues, allowing for observations in disease progression and treatment. These chip devices have the ability to help direct, and perhaps in the distant future even replace animal-based drug efficacy and toxicity studies, which have questionable relevance to human physiology. Here, we review recent developments in the in vitro modeling of barrier tissue interfaces with a focus on the use of novel and complex microfluidic device platforms.
Keywords: Barrier tissues; Drug discovery; In vitro modeling; Microfluidic technologies; Microphysiological systems; Organ-on-a-chip.
Figures


References
-
- Achyuta A.K., Conway A.J., Crouse R.B., Bannister E.C., Lee R.N., Katnik C.P., Behensky A.A., Cuevas J., Sundaram S.S. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip. 2013;13:542–553. - PubMed
-
- Astashkina A., Mann B., Grainger D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 2012;134:82–106. - PubMed
-
- Atac B., Wagner I., Horland R., Lauster R., Marx U., Tonevitsky A.G., Azar R.P., Lindner G. Skin and hair on-a-chip: in vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip. 2013;13:3555–3561. - PubMed
-
- Bellin M., Marchetto M.C., Gage F.H., Mummery C.L. Induced pluripotent stem cells: the new patient? Nat. Rev. Mol. Cell Biol. 2012;13:713–726. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

