Gut Microbiota Linked to Sexual Preference and HIV Infection
- PMID: 27077120
- PMCID: PMC4816837
- DOI: 10.1016/j.ebiom.2016.01.032
Gut Microbiota Linked to Sexual Preference and HIV Infection
Abstract
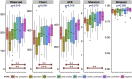
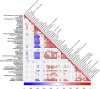
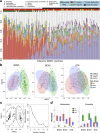
The precise effects of HIV-1 on the gut microbiome are unclear. Initial cross-sectional studies provided contradictory associations between microbial richness and HIV serostatus and suggested shifts from Bacteroides to Prevotella predominance following HIV-1 infection, which have not been found in animal models or in studies matched for HIV-1 transmission groups. In two independent cohorts of HIV-1-infected subjects and HIV-1-negative controls in Barcelona (n = 156) and Stockholm (n = 84), men who have sex with men (MSM) predominantly belonged to the Prevotella-rich enterotype whereas most non-MSM subjects were enriched in Bacteroides, independently of HIV-1 status, and with only a limited contribution of diet effects. Moreover, MSM had a significantly richer and more diverse fecal microbiota than non-MSM individuals. After stratifying for sexual orientation, there was no solid evidence of an HIV-specific dysbiosis. However, HIV-1 infection remained consistently associated with reduced bacterial richness, the lowest bacterial richness being observed in subjects with a virological-immune discordant response to antiretroviral therapy. Our findings indicate that HIV gut microbiome studies must control for HIV risk factors and suggest interventions on gut bacterial richness as possible novel avenues to improve HIV-1-associated immune dysfunction.
Keywords: 16S rDNA; Bacteroides; HIV-1; Microbiome; Microbiota; Prevotella.
Figures



Comment in
-
Effects of HIV, Immune Deficiency, and Confounding on the Distal Gut Microbiota.EBioMedicine. 2016 Jan 29;5:14-5. doi: 10.1016/j.ebiom.2016.01.034. eCollection 2016 Mar. EBioMedicine. 2016. PMID: 27077101 Free PMC article. No abstract available.
References
-
- Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R., Fernandes G.R., Tap J., Bruls T., Batto J.-M., Bertalan M., Borruel N., Casellas F., Fernandez L., Gautier L., Hansen T., Hattori M., Hayashi T., Kleerebezem M., Kurokawa K., Leclerc M., Levenez F., Manichanh C., Nielsen H.B., Nielsen T., Pons N., Poulain J., Qin J., Sicheritz-Ponten T., Tims S., Torrents D., Ugarte E., Zoetendal E.G., Wang J., Guarner F., Pedersen O., de Vos W.M., Brunak S., Doré J., Antolín M., Artiguenave F., Blottiere H.M., Almeida M., Brechot C., Cara C., Chervaux C., Cultrone A., Delorme C., Denariaz G., Dervyn R., Foerstner K.U., Friss C., van de Guchte M., Guedon E., Haimet F., Huber W., van Hylckama-Vlieg J., Jamet A., Juste C., Kaci G., Knol J., Lakhdari O., Layec S., Le Roux K., Maguin E., Mérieux A., Melo Minardi R., M'rini C., Muller J., Oozeer R., Parkhill J., Renault P., Rescigno M., Sanchez N., Sunagawa S., Torrejon A., Turner K., Vandemeulebrouck G., Varela E., Winogradsky Y., Zeller G., Weissenbach J., Ehrlich S.D., Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. - PMC - PubMed
-
- Brenchley J.M., Price D.A., Schacker T.W., Asher T.E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D., Blazar B.R., Rodriguez B., Teixeira-Johnson L., Landay A., Martin J.N., Hecht F.M., Picker L.J., Lederman M.M., Deeks S.G., Douek D.C. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. - PubMed
-
- Claesson M.J., Jeffery I.B., Conde S., Power S.E., O'Connor E.M., Cusack S., Harris H.M.B., Coakley M., Lakshminarayanan B., O'Sullivan O., Fitzgerald G.F., Deane J., O'Connor M., Harnedy N., O'Connor K., O'Mahony D., van Sinderen D., Wallace M., Brennan L., Stanton C., Marchesi J.R., Fitzgerald A.P., Shanahan F., Hill C., Ross R.P., O'Toole P.W. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. - PubMed
-
- Clarke S.F., Murphy E.F., O'Sullivan O., Lucey A.J., Humphreys M., Hogan A., Hayes P., O'Reilly M., Jeffery I.B., Wood-Martin R., Kerins D.M., Quigley E., Ross R.P., O'Toole P.W., Molloy M.G., Falvey E., Shanahan F., Cotter P.D. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1919. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

