The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis
- PMID: 27077231
- PMCID: PMC5338123
- DOI: 10.1111/bcp.12975
The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis
Abstract
Aims: Deprescribing is a suggested intervention to reverse the potential iatrogenic harms of inappropriate polypharmacy. The review aimed to determine whether or not deprescribing is a safe, effective and feasible intervention to modify mortality and health outcomes in older adults.
Methods: Specified databases were searched from inception to February 2015. Two researchers independently screened all retrieved articles for inclusion, assessed study quality and extracted data. Data were pooled using RevMan v5.3. Eligible studies included those where older adults had at least one medication deprescribed. The primary outcome was mortality. Secondary outcomes were adverse drug withdrawal events, psychological and physical health outcomes, quality of life, and medication usage (e.g. successful deprescribing, number of medications prescribed, potentially inappropriate medication use).
Results: A total of 132 papers met the inclusion criteria, which included 34 143 participants aged 73.8 ± 5.4 years. In nonrandomized studies, deprescribing polypharmacy was shown to significantly decrease mortality (OR 0.32, 95% CI: 0.17-0.60). However, this was not statistically significant in the randomized studies (OR 0.82, 95% CI 0.61-1.11). Subgroup analysis revealed patient-specific interventions to deprescribe demonstrated a significant reduction in mortality (OR 0.62, 95% CI 0.43-0.88). However, generalized educational programmes did not change mortality (OR 1.21, 95% CI 0.86-1.69).
Conclusions: Although nonrandomized data suggested that deprescribing reduces mortality, deprescribing was not shown to alter mortality in randomized studies. Mortality was significantly reduced when applying patient-specific interventions to deprescribe in randomized studies.
Keywords: deprescribing; medication discontinuation; meta-analysis; older adults; polypharmacy; systematic review.
© 2016 The British Pharmacological Society.
Figures
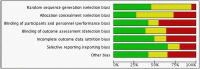
 Low risk of bias,
Low risk of bias,  unclear risk of bias,
unclear risk of bias,  high risk of bias
high risk of bias



References
-
- Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011; 10: 430–9. - PubMed
-
- Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta‐analysis involving more than 65,000 patients. J Am Coll Cardiol 2008; 52: 1769–81. - PubMed
-
- Home PD, Pocock SJ, Beck‐Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open‐label trial. Lancet 2009; 373: 2125–35. - PubMed
-
- Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta‐analysis on 11,000 participants from 42 trials. Am J Med 2009; 122: 290–300. - PubMed
-
- Roehrborn CG, Siami P, Barkin J, Damião R, Major‐Walker K, Nandy I, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4‐year results from the CombAT study. Eur Urol 2010; 57: 123–31. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

