Accelerated Lactate Dehydrogenase Activity Potentiates Osteoclastogenesis via NFATc1 Signaling
- PMID: 27077737
- PMCID: PMC4831772
- DOI: 10.1371/journal.pone.0153886
Accelerated Lactate Dehydrogenase Activity Potentiates Osteoclastogenesis via NFATc1 Signaling
Abstract
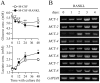
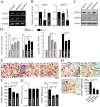
Osteoclasts seem to be metabolic active during their differentiation and bone-resorptive activation. However, the functional role of lactate dehydrogenase (LDH), a tetrameric enzyme consisting of an A and/or B subunit that catalyzes interconversion of pyruvate to lactate, in RANKL-induced osteoclast differentiation is not known. In this study, RANKL treatment induced gradual gene expression and activation of the LDH A2B2 isotype during osteoclast differentiation as well as the LDH A1B3 and B4 isotypes during osteoclast maturation after pre-osteoclast formation. Glucose consumption and lactate production in growth media were accelerated during osteoclast differentiation, together with enhanced expression of H+-lactate co-transporter and increased extracellular acidification, demonstrating that glycolytic metabolism was stimulated during differentiation. Further, oxygen consumption via mitochondria was stimulated during osteoclast differentiation. On the contrary, depletion of LDH-A or LDH-B subunit suppressed both glycolytic and mitochondrial metabolism, resulting in reduced mature osteoclast formation via decreased osteoclast precursor fusion and down-regulation of the osteoclastogenic critical transcription factor NFATc1 and its target genes. Collectively, our findings suggest that RANKL-induced LDH activation stimulates glycolytic and mitochondrial respiratory metabolism, facilitating mature osteoclast formation via osteoclast precursor fusion and NFATc1 signaling.
Conflict of interest statement
Figures


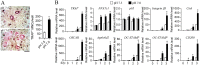
References
-
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289(5484):1508–14. . - PubMed
-
- Palokangas H, Mulari M, Vaananen HK. Endocytic pathway from the basal plasma membrane to the ruffled border membrane in bone-resorbing osteoclasts. Journal of cell science. 1997;110 (Pt 15):1767–80. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

