Endothelial to Mesenchymal Transition (EndoMT) in the Pathogenesis of Human Fibrotic Diseases
- PMID: 27077889
- PMCID: PMC4850468
- DOI: 10.3390/jcm5040045
Endothelial to Mesenchymal Transition (EndoMT) in the Pathogenesis of Human Fibrotic Diseases
Abstract
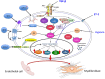
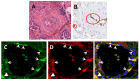
Fibrotic diseases encompass a wide spectrum of clinical entities including systemic fibrotic diseases such as systemic sclerosis, sclerodermatous graft versus host disease, nephrogenic systemic fibrosis, and IgG₄-associated sclerosing disease, as well as numerous organ-specific disorders including radiation-induced fibrosis, and cardiac, pulmonary, liver, and kidney fibrosis. Although their causative mechanisms are quite diverse, these diseases share the common feature of an uncontrolled and progressive accumulation of fibrous tissue macromolecules in affected organs leading to their dysfunction and ultimate failure. The pathogenesis of fibrotic diseases is complex and despite extensive investigation has remained elusive. Numerous studies have identified myofibroblasts as the cells responsible for the establishment and progression of the fibrotic process. Tissue myofibroblasts in fibrotic diseases originate from several sources including quiescent tissue fibroblasts, circulating CD34+ fibrocytes, and the phenotypic conversion of various cell types including epithelial and endothelial cells into activated myofibroblasts. However, the role of the phenotypic transition of endothelial cells into mesenchymal cells (Endothelial to Mesenchymal Transition or EndoMT) in the pathogenesis of fibrotic disorders has not been fully elucidated. Here, we review the evidence supporting EndoMT's contribution to human fibrotic disease pathogenesis.
Keywords: EndoMT; Endothelial Mesenchymal Transition; collagen; endothelial cell; extracellular matrix; fibrosis; fibrotic diseases; idiopathic pulmonary fibrosis; myofibroblast; systemic sclerosis; transforming growth factor-β.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

