Oxytocin for preventing postpartum haemorrhage (PPH) in non-facility birth settings
- PMID: 27078125
- PMCID: PMC8665833
- DOI: 10.1002/14651858.CD011491.pub2
Oxytocin for preventing postpartum haemorrhage (PPH) in non-facility birth settings
Abstract
Background: Postpartum haemorrhage (PPH) is the single leading cause of maternal mortality worldwide. Most of the deaths associated with PPH occur in resource-poor settings where effective methods of prevention and treatment - such as oxytocin - are not accessible because many births still occur at home, or in community settings, far from a health facility. Likewise, most of the evidence supporting oxytocin effectiveness comes from hospital settings in high-income countries, mainly because of the need of well-organised care for its administration and monitoring. Easier methods for oxytocin administration have been developed for use in resource-poor settings, but as far as we know, its effectiveness has not been assessed in a systematic review.
Objectives: To assess the effectiveness and safety of oxytocin provided in non-facility birth settings by any way in the third stage of labour to prevent PPH.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register, the WHO International Clinical Trials Registry Platform (ICTRP), ClinicalTrials.gov (12 November 2015), and reference lists of retrieved reports.
Selection criteria: All published, unpublished or ongoing randomised or quasi-randomised controlled trials comparing the administration of oxytocin with no intervention, or usual/standard care for the management of the third stage of labour in non-facility birth settings were considered for inclusion.Quasi-randomised controlled trials and randomised controlled trials published in abstract form only were eligible for inclusion but none were identified. Cross-over trials were not eligible for inclusion in this review.
Data collection and analysis: Two review authors independently assessed studies for eligibility, assessed risk of bias and extracted the data using an agreed data extraction form. Data were checked for accuracy.
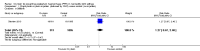
Main results: We included one cluster-randomised trial conducted in four rural districts in Ghana that randomised 28 community health officers (CHOs) (serving 2404 potentially eligible pregnant women) to the intervention group and 26 CHOs (serving 3515 potentially eligible pregnant women) to the control group. Overall, the trial had a high risk of bias. CHOs delivered the intervention in the experimental group (injection of 10 IU (international units) of oxytocin in the thigh one minute following birth using a prefilled, auto-disposable syringe). In the control group, CHOs did not provide this prophylactic injection to the women they observed. CHOs had no midwifery skills and did not in any way manage the birth. All other CHO activities (outcome measurement, data collection, and early treatment and referral when necessary) were identical across the control and oxytocin CHOs.Although only one of the nine cases of severe PPH (blood loss greater or equal to 1000 mL) occurred in the oxytocin group, the effect estimate for this outcome was very imprecise and it is uncertain whether the intervention prevents severe PPH (risk ratio (RR) 0.16, 95% confidence interval (CI) 0.02 to 1.30; 1570 women (very low-quality evidence)). Similarly, because of the lack of cases of severe maternal morbidity (e.g. uterine rupture) and maternal deaths, it was not possible to obtain effect estimates for those outcomes (both very low-quality evidence).Oxytocin compared with the control group decreased the incidence of PPH (> 500 mL) in both our unadjusted (RR 0.48, 95% CI 0.28 to 0.81; 1569 women) and adjusted (RR 0.49, 95% CI 0.27 to 0.90; 1174 women (both low-quality evidence)) analyses. There was little or no difference between the oxytocin and control groups on the rates of transfer or referral of the mother to a healthcare facility (RR 0.72, 95% CI 0.34 to 1.56; 1586 women (low-quality evidence)), stillbirths (RR 1.27, 95% CI 0.67 to 2.40; 2006 infants (low-quality evidence)); andearly infant deaths (0 to three days) (RR 1.03, 95% CI 0.35 to 3.07; 1969 infants (low-quality evidence)). There were no cases of needle-stick injury or any other maternal major or minor adverse event or unanticipated harmful event. There were no cases of oxytocin use during labour.There were no data reported for some of this review's secondary outcomes: manual removal of placenta, maternal anaemia, neonatal death within 28 days, neonatal transfer to health facility for advanced care, breastfeeding rates. Similarly, the women's or the provider's satisfaction with the intervention was not reported.
Authors' conclusions: It is uncertain if oxytocin administered by CHO in non-facility settings compared with a control group reduces the incidence of severe PPH (>1000 mL), severe maternal morbidity or maternal deaths. However, the intervention probably decreases the incidence of PPH (> 500 mL).The quality of the one trial included in this review was limited because of the risk of attrition and recruitment biases related to limitations in the follow-up of pregnant women in both arms of the trials and some baseline imbalance on the size of babies at birth. Additionally, there was serious imprecision of the effect estimates for most of the primary outcomes mainly because of the size of the trial, very few or no events and CIs around both relative and absolute estimates of effect that include both appreciable benefit and appreciable harm.Although the trial presented data both for primary and secondary outcomes, it seemed to be underpowered to detect differences in the primary outcomes that are the ones more relevant for making judgments about the potential applicability of the intervention in other settings (especially severe PPH).Therefore, taking into account the extreme setting where the intervention was implemented, the limited role of the CHO in the trial and the lack of power for detecting effects on primary (relevant) outcomes, the applicability of the evidence found seems to be rather limited.Further well-executed and adequately-powered randomised controlled trials assessing the effects of using oxytocin in pre-filled injection devices or other new delivery systems (spray-dried ultrafine formulation of oxytocin) on severe PPH are urgently needed. Likewise, other important outcomes like possible adverse events and acceptability of the intervention by mothers and other community stakeholders should also be assessed.
Conflict of interest statement
TP: My institution has received funding from the Cochrane Global Evidence Synthesis Initiative to perform the work related with this review. I am also an editor with the Cochrane Effective Practice and Organisation of Care (EPOC) group. EA: none known EC: none known CV: My institution has received funding from the Cochrane Global Evidence Synthesis Initiative to perform the work related with this review. VS: My institution has received funding from the Cochrane Global Evidence Synthesis Initiative to perform the work related with this review.
Figures
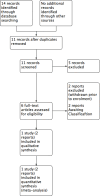









Update of
References
References to studies included in this review
Stanton 2013 {published data only}
-
- Stanton CK, Newton S, Mullany LC, Cofie P, Agyemang CT, Adiibokah E, et al. Impact on postpartum hemorrhage of prophylactic administration of oxytocin 10 IU via Uniject by peripheral health care providers at home births: Design of a community‐based cluster‐randomized trial. BMC Pregnancy and Childbirth 2012;12:42. - PMC - PubMed
References to studies excluded from this review
NCT01487278 {published data only}
-
- NCT01487278. Comparing misoprostol and oxytocin in UnijectTM postpartum hemorrhage (PPH) prevention in Mali. ClinicalTrials.gov (https://clinicaltrials.gov/) [accessed 7 April 2015] 2011.
Stanton 2010 {published data only}
-
- Stanton C. Effectiveness, safety and feasibility of auxiliary nurse midwives' (ANM) use of Oxytocin in uniject™ to prevent postpartum hemorrhage in India. ClinicalTrials.gov (https://clinicaltrials.gov/) [accessed 7 April 2015] 2010.
References to studies awaiting assessment
NCT01710566 {published data only}
-
- NCT01710566. Misoprostol and oxytocin in Uniject® for postpartum hemorrhage prevention in communities. ClinicalTrials.gov (https://clinicaltrials.gov/) [accessed 7 April 2015] 2012.
NCT01713153 {published data only}
-
- NCT01713153. Comparing misoprostol and oxytocin in uniject for postpartum hemorrhage (PPH) prevention in Senegal. ClinicalTrials.gov (https://clinicaltrials.gov/) [accessed 7 April 2015] 2012.
Additional references
Althabe 2011
-
- Althabe F, Mazzoni A, Cafferata ML, Gibbons L, Karolinski A, Armbruster D, et al. Using Uniject to increase the use of prophylactic oxytocin for management of the third stage of labor in Latin America. International Journal of Gynecology & Obstetrics 2011;114(2):184‐9. - PubMed
Amico 1987
-
- Amico JA, Ulbrecht JS, Robinson AG. Clearance studies of oxytocin in humans using radioimmunoassay measurements of the hormone in plasma and urine. Journal of Clinical Endocrinology and Metabolism 1987;64(2):340‐5. - PubMed
Amsalem 2014
-
- Amsalem H, Aldrich CJ, Oskamp M, Windrim R, Farine D. Postpartum uterine response to oxytocin and carbetocin. Journal of Reproductive Medicine 2014;59(3‐4):167‐73. - PubMed
Begley 2015
Bhattacharya 2013
Breathnach 2006
-
- Breathnach F, Geary M. Standard medical therapy. In: B‐Lynch C, Keith LG, Lalonde AB, Karoshi M editor(s). A Textbook of Postpartum Haemorrhage. Sapiens Publishing, 2006.
Carroli 2008
-
- Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Practice & Research Clinical Obstetrics & Gynaecology 2008;22:999‐1012. - PubMed
Chu 2012
Crowe 2012
Darmstadt 2009
Dyer 2011
-
- Dyer RA, Butwick AJ, Carvalho B. Oxytocin for labour and caesarean delivery: implications for the anaesthesiologist. Current Opinion in Anesthesiology 2011;24(3):255‐61. - PubMed
Evans 1997
-
- Evans JJ. Oxytocin in the human‐‐regulation of derivations and destinations. European Journal of Endocrinology 1997;137(6):559‐71. - PubMed
Gonser 1995
-
- Gonser M. Labor induction and augmentation with oxytocin: pharmacokinetic considerations. Archives of Gynecology and Obstetrics 1995;256(2):63‐6. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2013
Hundley 2013
-
- Hundley VA, Avan BI, Sullivan CJ, Graham WJ. Should oral misoprostol be used to prevent postpartum haemorrhage in home‐birth settings in low‐resource countries? A systematic review of the evidence. BJOG: an international journal of obstetrics and gynaecology 2013;120:277‐85; discussion 86‐7. - PubMed
ICM‐FIGO 2003
-
- ICM/FIGO Joint Statement. Management of the Third Stage of Labour to Prevent Post‐Partum Haemorrhage. http://www.figo.org/files/figo‐corp/docs/PPH%20Joint%20Statement.pdf [accessed 4 August 2014] 2003.
In 2011
Jangsten 2005
-
- Jangsten E, Strand R, Gomez de Freitas EG, Hellstrom AL, Johansson A, Bergstrom S. Women's perceptions of pain and discomfort after childbirth in Angola. African Journal of Reproductive Health 2005;9(3):148‐58. - PubMed
Karoshi 2009
-
- Karoshi M, Keith L. Challenges in managing postpartum hemorrhage in resource‐poor countries. Clinical Obstetrics and Gynecology 2009;52(2):285‐98. - PubMed
Kassebaum 2014
Millard 2014
-
- Millard C, Bhrlikova P, Pollock A. Commentary: Evidence versus influence in the WHO procedure for approving essential medicines: misoprostol for maternal health. BMJ 2014;349:g4823. - PubMed
Miller 2004
-
- Miller S, Lester F, Hensleigh P. Prevention and treatment of postpartum hemorrhage: new advances for low‐resource settings. Journal of Midwifery & Women's Health 2004;49(4):283‐92. - PubMed
Mousa 2014
NICE 2007
-
- National Institute for Health and Clinical Excellence. Normal labour: third stage. Intrapartum Care: Care of Healthy Women and Their Babies During Childbirth, NICE Guideline 55. London: National Institute for Health and Clinical Excellence, 2007. - PubMed
Oladapo 2012a
Oladapo 2012b
Pichon‐Riviere 2015
-
- Pichon‐Riviere A, Glujovsky D, Garay OU, Augustovski F, Ciapponi A, Serpa M, et al. Oxytocin in Uniject disposable auto‐disable injection system versus standard use for the prevention of postpartum hemorrhage in Latin America and the Caribbean: a cost‐effectiveness analysis. PLoS One 2015;10(6):e0129044. - PMC - PubMed
Prankerd 2013
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ronsmans 2006
-
- Ronsmans C, Graham WJ, Lancet Maternal Survival Series Steering Group. Maternal mortality: who, when, where, and why. Lancet 2006;368:1189‐200. - PubMed
Say 2014
-
- Say L, Chou D, Gemmill A, Tuncalp O, Moller A, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Golbal Health 2014;2(6):e323‐e333. - PubMed
Seitchik 1984
-
- Seitchik J, Amico J, Robinson AG, Castillo M. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics. American Journal of Obstetrics and Gynecology 1984;150(3):225‐8. - PubMed
Sheldon 2013
Stanton 2012
-
- Stanton CK, Newton S, Mullany LC, Cofie P, Agyemang CT, Adiibokah E, et al. Impact on postpartum hemorrhage of prophylactic administration of oxytocin 10 IU via Uniject by peripheral health care providers at home births: design of a community‐based cluster‐randomized trial. BMC Pregnancy and Childbirth 2012;12:42. - PMC - PubMed
Strand 2005
-
- Strand RT, Silva F, Jangsten E, Bergstrom S. Postpartum hemorrhage: a prospective, comparative study in Angola using a new disposable device for oxytocin administration. Acta Obstetricia et Gynecologica Scandinavica 2005;84(3):260‐5. - PubMed
Svanström 2008
-
- Svanström MC, Biber B, Hanes M, Johansson G, Näslund U, Bålfors EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double‐blind comparison of oxytocin and methylergometrine during caesarean section. British Journal of Anaesthesia 2008;100(5):683‐9. - PubMed
Tsu 2003
-
- Tsu VD, Sutanto A, Vaidya K, Coffey P, Widjaya A. Oxytocin in prefilled Uniject injection devices for managing third‐stage labor in Indonesia. International Journal of Gynecology & Obstetrics 2003;83(1):103‐11. - PubMed
UN 2000
-
- United Nations. United Nations Millennium Declaration. U.N. document A/RES/55/2, adopted September 8. http://www.un.org/millennium/declaration/ares552e.htm [accessed September 2014] 2000.
Westhoff 2013
WHO 1993
-
- World Health Organization. Stability of Injectable Oxytocics in Tropical Climates: Results of Field Surveys and Simulation Studies on Ergometrine, Methylergometrine and Oxytocin. EDM Research Series No 008. Geneva: WHO, 1993.
WHO 2007
-
- World Health Organization. Guidelines for the Prevention of Postpartum Haemorrhage. Geneva: WHO, 2007.
WHO 2012
-
- World Health Organization, Department of Reproductive Health and Research. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: WHO, 2012. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

