Imaging of Liver Tumors Using Surface-Enhanced Raman Scattering Nanoparticles
- PMID: 27078225
- PMCID: PMC4884645
- DOI: 10.1021/acsnano.5b07200
Imaging of Liver Tumors Using Surface-Enhanced Raman Scattering Nanoparticles
Abstract
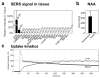
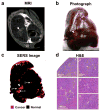
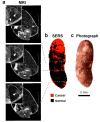
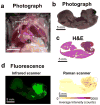
Complete surgical resection is the ideal first-line treatment for most liver malignancies. This goal would be facilitated by an intraoperative imaging method that enables more precise visualization of tumor margins and detection of otherwise invisible microscopic lesions. To this end, we synthesized silica-encapsulated surface-enhanced Raman scattering (SERS) nanoparticles (NPs) that act as a molecular imaging agent for liver malignancies. We hypothesized that, after intravenous administration, SERS NPs would avidly home to healthy liver tissue but not to intrahepatic malignancies. We tested these SERS NPs in genetically engineered mouse models of hepatocellular carcinoma and histiocytic sarcoma. After intravenous injection, liver tumors in both models were readily identifiable with Raman imaging. In addition, Raman imaging using SERS NPs enabled detection of microscopic lesions in liver and spleen. We compared the performance of SERS NPs to fluorescence imaging using indocyanine green (ICG). We found that SERS NPs delineate tumors more accurately and are less susceptible to photobleaching. Given the known advantages of SERS imaging, namely, high sensitivity and specific spectroscopic detection, these findings hold promise for improved resection of liver cancer.
Keywords: hepatocellular carcinoma; image-guided tumor resection; intraoperative imaging; nanoparticles; sarcoma; surface-enhanced Raman scattering.
Figures





References
-
- Ishizawa T, Gumbs AA, Kokudo N, Gayet B. Laparoscopic Segmentectomy of the Liver: From Segment I to VIII. Ann Surg. 2012;256:959–964. - PubMed
-
- Kudo H, Ishizawa T, Tani K, Harada N, Ichida A, Shimizu A, Kaneko J, Aoki T, Sakamoto Y, Sugawara Y, Hasegawa K, Kokudo N. Visualization of Subcapsular Hepatic Malignancy by Indocyanine-Green Fluorescence Imaging During Laparoscopic Hepatectomy. Surg Endosc. 2014;28:2504–2508. - PubMed
-
- Bonanni L, De’liguori Carino N, Deshpande R, Ammori BJ, Sherlock DJ, Valle JW, Tam E, O’Reilly DA. A Comparison of Diagnostic Imaging Modalities for Colorectal Liver Metastases. Eur J Surg Oncol. 2014;40:545–550. - PubMed
-
- Grimm J, Kircher MF, Weissleder R. Cell Tracking. Principles and Applications. Radiologe. 2007;47:25–33. - PubMed
-
- Kircher MF, Gambhir SS, Grimm J. Noninvasive Cell-Tracking Methods. Nat Rev Clin Oncol. 2011;8:677–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

