Serum uPAR as Biomarker in Breast Cancer Recurrence: A Mathematical Model
- PMID: 27078836
- PMCID: PMC4831695
- DOI: 10.1371/journal.pone.0153508
Serum uPAR as Biomarker in Breast Cancer Recurrence: A Mathematical Model
Abstract
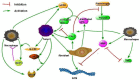
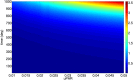
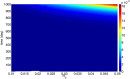
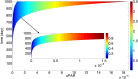
There are currently over 2.5 million breast cancer survivors in the United States and, according to the American Cancer Society, 10 to 20 percent of these women will develop recurrent breast cancer. Early detection of recurrence can avoid unnecessary radical treatment. However, self-examination or mammography screening may not discover a recurring cancer if the number of surviving cancer cells is small, while biopsy is too invasive and cannot be frequently repeated. It is therefore important to identify non-invasive biomarkers that can detect early recurrence. The present paper develops a mathematical model of cancer recurrence. The model, based on a system of partial differential equations, focuses on tissue biomarkers that include the plasminogen system. Among them, only uPAR is known to have significant correlation to its concentration in serum and could therefore be a good candidate for serum biomarker. The model includes uPAR and other associated cytokines and cells. It is assumed that the residual cancer cells that survived primary cancer therapy are concentrated in the same location within a region with a very small diameter. Model simulations establish a quantitative relation between the diameter of the growing cancer and the total uPAR mass in the cancer. This relation is used to identify uPAR as a potential serum biomarker for breast cancer recurrence.
Conflict of interest statement
Figures


References
-
- Foundation BCR. Breast cancer statistics & resources. 2016.
-
- Foundation USBCR. U.S. Breast cancer statistics. 2016.
-
- Kantelhardt EJ, Vetter M, Schmidt M, Veyret C, Augustin D, et al. Prospective evaluation of prognostic factors uPA/PAI-1 in node-negative breast cancer: phase III NNBC3-Europe trial (AGO, GBG, EORTC-PBG) comparing FEC versus FECDocetaxel. BMC Cancer. 2011;11:140 10.1186/1471-2407-11-140 - DOI - PMC - PubMed
-
- Rubinstein WS, O’Neill SM, Peters JA, Rittmeyer LJ, Stadler MP. Mathematical modeling for breast cancer risk assessment. State of the art and role in medicine. Oncology (Williston Park, NY). 2002;16(8):1082–1094. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

