CRISPR-Cas9(D10A) nickase-based genotypic and phenotypic screening to enhance genome editing
- PMID: 27079678
- PMCID: PMC4832145
- DOI: 10.1038/srep24356
CRISPR-Cas9(D10A) nickase-based genotypic and phenotypic screening to enhance genome editing
Abstract
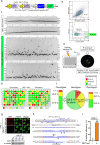
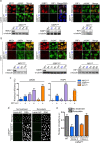
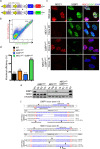
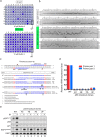
The RNA-guided Cas9 nuclease is being widely employed to engineer the genomes of various cells and organisms. Despite the efficient mutagenesis induced by Cas9, off-target effects have raised concerns over the system's specificity. Recently a "double-nicking" strategy using catalytic mutant Cas9(D10A) nickase has been developed to minimise off-target effects. Here, we describe a Cas9(D10A)-based screening approach that combines an All-in-One Cas9(D10A) nickase vector with fluorescence-activated cell sorting enrichment followed by high-throughput genotypic and phenotypic clonal screening strategies to generate isogenic knockouts and knock-ins highly efficiently, with minimal off-target effects. We validated this approach by targeting genes for the DNA-damage response (DDR) proteins MDC1, 53BP1, RIF1 and P53, plus the nuclear architecture proteins Lamin A/C, in three different human cell lines. We also efficiently obtained biallelic knock-in clones, using single-stranded oligodeoxynucleotides as homologous templates, for insertion of an EcoRI recognition site at the RIF1 locus and introduction of a point mutation at the histone H2AFX locus to abolish assembly of DDR factors at sites of DNA double-strand breaks. This versatile screening approach should facilitate research aimed at defining gene functions, modelling of cancers and other diseases underpinned by genetic factors, and exploring new therapeutic opportunities.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

