Abnormal Population Responses in the Somatosensory Cortex of Alzheimer's Disease Model Mice
- PMID: 27079783
- PMCID: PMC4832196
- DOI: 10.1038/srep24560
Abnormal Population Responses in the Somatosensory Cortex of Alzheimer's Disease Model Mice
Abstract
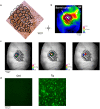
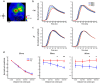
Alzheimer's disease (AD) is the most common form of dementia. One of the neuropathological hallmarks of AD is the accumulation of amyloid-β plaques. Overexpression of human amyloid precursor protein in transgenic mice induces hippocampal and neocortical amyloid-β accumulation and plaque deposition that increases with age. The impact of these effects on neuronal population responses and network activity in sensory cortex is not well understood. We used Voltage Sensitive Dye Imaging, to investigate at high spatial and temporal resolution, the sensory evoked population responses in the barrel cortex of aged transgenic (Tg) mice and of age-matched non-transgenic littermate controls (Ctrl) mice. We found that a whisker deflection evoked abnormal sensory responses in the barrel cortex of Tg mice. The response amplitude and the spatial spread of the cortical responses were significantly larger in Tg than in Ctrl mice. At the network level, spontaneous activity was less synchronized over cortical space than in Ctrl mice, however synchronization during evoked responses induced by whisker deflection did not differ between the two groups. Thus, the presence of elevated Aβ and plaques may alter population responses and disrupts neural synchronization in large-scale networks, leading to abnormalities in sensory processing.
Figures


References
-
- Hardy J. & Selkoe D. J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science (80−) 297, 353–356 (2002). - PubMed
-
- Karran E., Mercken M. & Strooper B. De. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 10, 698–712 (2011). - PubMed
-
- Masliah E. et al. Synaptic and neuritic alterations during the progression of Alzheimer’s disease. Neurosci. Lett. 174, 67–72 (1994). - PubMed
-
- Adalbert R. et al. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain 132, 402–416 (2009). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

