An Intronic Flk1 Enhancer Directs Arterial-Specific Expression via RBPJ-Mediated Venous Repression
- PMID: 27079877
- PMCID: PMC4894770
- DOI: 10.1161/ATVBAHA.116.307517
An Intronic Flk1 Enhancer Directs Arterial-Specific Expression via RBPJ-Mediated Venous Repression
Abstract
Objective: The vascular endothelial growth factor (VEGF) receptor Flk1 is essential for vascular development, but the signaling and transcriptional pathways by which its expression is regulated in endothelial cells remain unclear. Although previous studies have identified 2 Flk1 regulatory enhancers, these are dispensable for Flk1 expression, indicating that additional enhancers contribute to Flk1 regulation in endothelial cells. In the present study, we sought to identify Flk1 enhancers contributing to expression in endothelial cells.
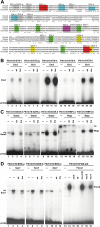
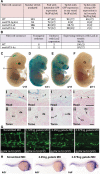
Approach and results: A region of the 10th intron of the Flk1 gene (Flk1in10) was identified as a putative enhancer and tested in mouse and zebrafish transgenic models. This region robustly directed reporter gene expression in arterial endothelial cells. Using a combination of targeted mutagenesis of transcription factor-binding sites and gene silencing of transcription factors, we found that Gata and Ets factors are required for Flk1in10 enhancer activity in all endothelial cells. Furthermore, we showed that activity of the Flk1in10 enhancer is restricted to arteries through repression of gene expression in venous endothelial cells by the Notch pathway transcriptional regulator Rbpj.
Conclusions: This study demonstrates a novel mechanism of arterial-venous identity acquisition, indicates a direct link between the Notch and VEGF signaling pathways, and illustrates how cis-regulatory diversity permits differential expression outcomes from a limited repertoire of transcriptional regulators.
Keywords: arterial-venous specification; artery; endothelial cells; mice; notch; veins; zebrafish.
© 2016 The Authors.
Figures




References
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. - PubMed
-
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. - PubMed
-
- Sacilotto N, Monteiro R, Fritzsche M, Becker PW, Sanchez-Del-Campo L, Liu K, Pinheiro P, Ratnayaka I, Davies B, Goding CR, Patient R, Bou-Gharios G, De Val S. Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci USA. 2013;110:11893–11898. doi: 10.1073/pnas.1300805110. - PMC - PubMed
-
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

