A Comparison of 3 Vitamin D Dosing Regimens in Extremely Preterm Infants: A Randomized Controlled Trial
- PMID: 27079965
- PMCID: PMC4925243
- DOI: 10.1016/j.jpeds.2016.03.028
A Comparison of 3 Vitamin D Dosing Regimens in Extremely Preterm Infants: A Randomized Controlled Trial
Abstract
Objective: To determine the optimal dose of vitamin D supplementation to achieve biochemical vitamin D sufficiency in extremely low gestational age newborns in a masked randomized controlled trial.
Study design: 100 infants 23 0/7-27 6/7 weeks gestation were randomized to vitamin D intakes of placebo (n = 36), 200 IU (n = 34), and 800 IU/d (n = 30) (approximating 200, 400, or 1000 IU/d, respectively, when vitamin D routinely included in parenteral or enteral nutrition is included). The primary outcomes were serum 25-hydroxy vitamin D concentrations on postnatal day 28 and the number of days alive and off respiratory support in the first 28 days.
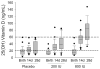
Results: At birth, 67% of infants had 25-hydroxy vitamin D <20 ng/mL suggesting biochemical vitamin D deficiency. Vitamin D concentrations on day 28 were (median [25th-75th percentiles], ng/mL): placebo: 22 (13-47), 200 IU: 39 (26-57), 800 IU: 84.5 (52-99); P < .001. There were no differences in days alive and off respiratory support (median [25th-75th percentiles], days): placebo: 1 (0-11), 200 IU: 0 (0-8), and 800 IU: 0.5 (0-22); P = .63, or other respiratory outcomes among groups.
Conclusions: At birth, most extremely preterm infants have biochemical vitamin D deficiency. This biochemical deficiency is reduced on day 28 by supplementation with 200 IU/d and prevented by 800 IU/d. Larger trials are required to determine if resolution of biochemical vitamin D deficiency improves clinical outcomes.
Trial registration: ClinicalTrials.gov: NCT01600430.
Keywords: bronchopulmonary dysplasia; intraventricular hemorrhage; necrotizing enterocolitis; premature infant; randomized trial; retinopathy of prematurity; septicemia.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
References
-
- Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. American journal of respiratory and critical care medicine. 2011;183:1336–43. - PubMed
-
- Nguyen T, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. cell differentiation. 1996;28:36. - PubMed
-
- Phokela SS, Peleg S, Moya FR, Alcorn JL. Regulation of human pulmonary surfactant protein gene expression by 1α, 25-dihydroxyvitamin D3. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2005;289:L617–L26. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

