Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery
- PMID: 27080226
- PMCID: PMC5153580
- DOI: 10.1038/cgt.2016.14
Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery
Abstract
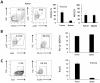
Metastatic spread of colorectal cancer (CRC) to the peritoneal cavity is common and difficult to treat, with many patients dying from malignant bowel obstruction. Chimeric antigen receptor T cell (CAR-T) immunotherapy has shown great promise, and we previously reported murine and phase I clinical studies on regional intrahepatic CAR-T infusion for CRC liver metastases. We are now studying intraperitoneal (IP) delivery of CAR-Ts for peritoneal carcinomatosis. Regional IP infusion of CAR-T resulted in superior protection against carcinoembryonic antigen (CEA+) peritoneal tumors, when compared with systemically infused CAR-Ts. IP CAR-Ts also provided prolonged protection against IP tumor re-challenges and demonstrated an increase in effector memory phenotype over time. IP CAR-Ts provided protection against tumor growth at distant subcutaneous (SC) sites in association with increases in serum IFNγ levels. Given the challenges posed by immunoinhibitory pathways in solid tumors, we combined IP CAR-T treatment with suppressor cell targeting. High frequencies of myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg) were found within the IP tumors, with MDSC expressing high levels of immunosuppressive PD-L1. Combinatorial IP CAR-T treatment with depleting antibodies against MDSC and Treg further improved efficacy against peritoneal metastases. Our data support further development of combinatorial IP CAR-T immunotherapy for peritoneal malignancies.
Figures

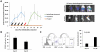


References
-
- Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–43. - PubMed
-
- Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–32. - PubMed
-
- Cao C, Yan TD, Black D, Morris DL. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2009;16(8):2152–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

