Fracture and Growth Are Competing Forces Determining the Fate of Conformers in Tau Fibril Populations
- PMID: 27080260
- PMCID: PMC4933275
- DOI: 10.1074/jbc.M116.715557
Fracture and Growth Are Competing Forces Determining the Fate of Conformers in Tau Fibril Populations
Abstract
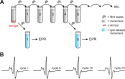
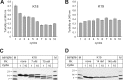
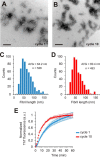
Tau fibrils are pathological aggregates that can transfer between neurons and then recruit soluble Tau monomers by template-assisted conversion. The propagation of different fibril polymorphs is thought to be a contributing factor to phenotypic diversity in Alzheimer disease and other Tauopathies. We found that a homogeneous population of Tau fibrils composed of the truncated version K18 (residues 244-372) gradually converted to a new set of fibril conformers when subjected to multiple cycles of seeding and growth. Using double electron-electron resonance (DEER) spectroscopy, we observed that the distances between spin labels at positions 311 and 328 in the fibril core progressively decreased. The findings were corroborated by changes in turbidity, morphology, and protease sensitivity. Fibrils that were initially formed under stirring conditions exhibited an increased fragility compared with fibrils formed quiescently after multiple cycles of seeding. The quiescently formed fibrils were marked by accelerated growth. The difference in fragility and growth between the different conformers explains how the change in incubation condition could lead to the amplification of a minor subpopulation of fibrils. Under quiescent conditions where fibril breakage is minimal, faster growing fibrils have a selective advantage. The findings are of general importance as they suggest that changes in selective pressures during fibril propagation in the human brain could result in the emergence of new fibril conformers with varied clinicopathological consequences.
Keywords: Alzheimer disease; Tau protein (Tau); aggregation; amyloid; electron paramagnetic resonance (EPR); fibril; prion.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
Spin Labeling and Characterization of Tau Fibrils Using Electron Paramagnetic Resonance (EPR).Methods Mol Biol. 2016;1345:185-99. doi: 10.1007/978-1-4939-2978-8_12. Methods Mol Biol. 2016. PMID: 26453213
-
Structure-based inhibitors halt prion-like seeding by Alzheimer's disease-and tauopathy-derived brain tissue samples.J Biol Chem. 2019 Nov 1;294(44):16451-16464. doi: 10.1074/jbc.RA119.009688. Epub 2019 Sep 19. J Biol Chem. 2019. PMID: 31537646 Free PMC article.
-
Structural disorder in four-repeat Tau fibrils reveals a new mechanism for barriers to cross-seeding of Tau isoforms.J Biol Chem. 2018 Nov 9;293(45):17336-17348. doi: 10.1074/jbc.RA118.005316. Epub 2018 Sep 21. J Biol Chem. 2018. PMID: 30242125 Free PMC article.
-
Amyloidogenesis of Tau protein.Protein Sci. 2017 Nov;26(11):2126-2150. doi: 10.1002/pro.3275. Epub 2017 Sep 13. Protein Sci. 2017. PMID: 28833749 Free PMC article. Review.
-
Tau strains shape disease.Acta Neuropathol. 2021 Jul;142(1):57-71. doi: 10.1007/s00401-021-02301-7. Epub 2021 Apr 8. Acta Neuropathol. 2021. PMID: 33830330 Free PMC article. Review.
Cited by
-
Molecular Crowding: The History and Development of a Scientific Paradigm.Chem Rev. 2024 Mar 27;124(6):3186-3219. doi: 10.1021/acs.chemrev.3c00615. Epub 2024 Mar 11. Chem Rev. 2024. PMID: 38466779 Free PMC article. Review.
-
The elusive tau molecular structures: can we translate the recent breakthroughs into new targets for intervention?Acta Neuropathol Commun. 2019 Mar 1;7(1):31. doi: 10.1186/s40478-019-0682-x. Acta Neuropathol Commun. 2019. PMID: 30823892 Free PMC article. Review.
-
The role of annealing and fragmentation in human tau aggregation dynamics.J Biol Chem. 2019 Mar 29;294(13):4728-4737. doi: 10.1074/jbc.RA118.006943. Epub 2019 Feb 11. J Biol Chem. 2019. PMID: 30745358 Free PMC article.
-
Distinct Conformations, Aggregation and Cellular Internalization of Different Tau Strains.Front Cell Neurosci. 2019 Jul 9;13:296. doi: 10.3389/fncel.2019.00296. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31338022 Free PMC article.
-
Amyloidogenic cross-seeding of Tau protein: Transient emergence of structural variants of fibrils.PLoS One. 2018 Jul 19;13(7):e0201182. doi: 10.1371/journal.pone.0201182. eCollection 2018. PLoS One. 2018. PMID: 30024984 Free PMC article.
References
-
- Lee V. M., Goedert M., and Trojanowski J. Q. (2001) Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159 - PubMed
-
- Spillantini M. G., and Goedert M. (2013) Tau pathology and neurodegeneration. Lancet Neurol. 12, 609–622 - PubMed
-
- Braak H., and Braak E. (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 - PubMed
-
- Verny M., Duyckaerts C., Agid Y., and Hauw J. J. (1996) The significance of cortical pathology in progressive supranuclear palsy. Clinico-pathological data in 10 cases. Brain 119, 1123–1136 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

