Psyllium Fiber Reduces Abdominal Pain in Children With Irritable Bowel Syndrome in a Randomized, Double-Blind Trial
- PMID: 27080737
- PMCID: PMC5064811
- DOI: 10.1016/j.cgh.2016.03.045
Psyllium Fiber Reduces Abdominal Pain in Children With Irritable Bowel Syndrome in a Randomized, Double-Blind Trial
Abstract
Background & aims: We sought to determine the efficacy of psyllium fiber treatment on abdominal pain and stool patterns in children with irritable bowel syndrome (IBS). We evaluated effects on breath hydrogen and methane production, gut permeability, and microbiome composition. We also investigated whether psychological characteristics of children or parents affected the response to treatment.
Methods: We performed a randomized, double-blind trial of 103 children (mean age, 13 ± 3 y) with IBS seen at primary or tertiary care settings. After 2 weeks on their habitual diet, children began an 8-day diet excluding carbohydrates thought to cause symptoms of IBS. Children with ≥75% improvement in abdominal pain were excluded (n = 17). Children were assigned randomly to groups given psyllium (n = 37) or placebo (maltodextrin, n = 47) for 6 weeks. Two-week pain and stool diaries were compared at baseline and during the final 2 weeks of treatment. We assessed breath hydrogen and methane production, intestinal permeability, and the composition of the microbiome before and after administration of psyllium or placebo. Psychological characteristics of children were measured at baseline.
Results: Children in the psyllium group had a greater reduction in the mean number of pain episodes than children in the placebo group (mean reduction of 8.2 ± 1.2 after receiving psyllium vs mean reduction of 4.1 ± 1.3 after receiving placebo; P = .03); the level of pain intensity did not differ between the groups. Psychological characteristics were not associated with response. At the end of the study period, the percentage of stools that were normal (Bristol scale scores, 3-5), breath hydrogen or methane production, intestinal permeability, and microbiome composition were similar between groups.
Conclusions: Psyllium fiber reduced the number of abdominal pain episodes in children with IBS, independent of psychological factors. Psyllium did not alter breath hydrogen or methane production, gut permeability, or microbiome composition. ClinicalTrials.gov no: NCT00526903.
Keywords: Abdominal Pain; Fiber; Irritable Bowel Syndrome; Microbiome; Psyllium.
Copyright © 2017 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures
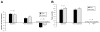
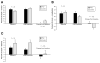
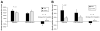

Comment in
-
Should We Treat Our Pediatric Irritable Bowel Syndrome Patients With Psyllium?Clin Gastroenterol Hepatol. 2016 Nov;14(11):1667. doi: 10.1016/j.cgh.2016.06.001. Epub 2016 Jun 7. Clin Gastroenterol Hepatol. 2016. PMID: 27283796 No abstract available.
-
Reply.Clin Gastroenterol Hepatol. 2016 Nov;14(11):1667-1668. doi: 10.1016/j.cgh.2016.06.021. Epub 2016 Jul 1. Clin Gastroenterol Hepatol. 2016. PMID: 27377875 No abstract available.
References
-
- Saps M, Seshadri R, Sztainberg M, et al. A prospective school-based study of abdominal pain and other common somatic complaints in children. J Pediatr. 2009;154:322–326. - PubMed
-
- Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1367–1374. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

