The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease
- PMID: 27081111
- PMCID: PMC4834858
- DOI: 10.1161/CIRCRESAHA.116.307509
The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease
Abstract
Nearly one-third of deaths in the United States are caused by cardiovascular disease (CVD) each year. In the past, CVD was thought to mainly affect men, leading to the exclusion of women and female animals from clinical studies and preclinical research. In light of sexual dimorphisms in CVD, a need exists to examine baseline cardiac differences in humans and the animals used to model CVD. In humans, sex differences are apparent at every level of cardiovascular physiology from action potential duration and mitochondrial energetics to cardiac myocyte and whole-heart contractile function. Biological sex is an important modifier of the development of CVD with younger women generally being protected, but this cardioprotection is lost later in life, suggesting a role for estrogen. Although endogenous estrogen is most likely a mediator of the observed functional differences in both health and disease, the signaling mechanisms involved are complex and are not yet fully understood. To investigate how sex modulates CVD development, animal models are essential tools and should be useful in the development of therapeutics. This review will focus on describing the cardiovascular sexual dimorphisms that exist both physiologically and in common animal models of CVD.
Keywords: cardiovascular diseases; estrogens; gonadal steroid hormones; heart failure; sex characteristics.
© 2016 American Heart Association, Inc.
Figures
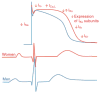

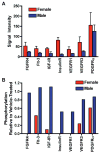

References
-
- Writing Group M; Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. - PubMed
-
- Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK American Heart A. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. Journal of the American College of Cardiology. 2011;57:1404–23. - PMC - PubMed
-
- Kelsey SF, James M, Holubkov AL, Holubkov R, Cowley MJ, Detre KM. Results of percutaneous transluminal coronary angioplasty in women. 1985–1986 National Heart, Lung, and Blood Institute’s Coronary Angioplasty Registry. Circulation. 1993;87:720–7. - PubMed
-
- Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G, Investigators W. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. Journal of the American College of Cardiology. 2006;47:S4–S20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

