Differential Receptor Tyrosine Kinase PET Imaging for Therapeutic Guidance
- PMID: 27081168
- PMCID: PMC5093919
- DOI: 10.2967/jnumed.115.169417
Differential Receptor Tyrosine Kinase PET Imaging for Therapeutic Guidance
Abstract
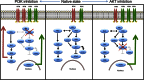
Inhibitors of the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway hold promise for the treatment of breast cancer, but resistance to these treatments can arise via feedback loops that increase surface expression of the receptor tyrosine kinases (RTK) epidermal growth factor receptor 1 (EGFR) and human epidermal growth factor receptor 3 (HER3), leading to persistent growth pathway signaling. We developed PET probes that provide a method of imaging this response in vivo, determining which tumors may use this escape pathway while avoiding the need for repeated biopsies.
Methods: Anti-EGFR-F(ab')2 and anti-HER3-F(ab')2 were generated from monoclonal antibodies by enzymatic digestion, conjugated to DOTA, and labeled with (64)Cu. A panel of breast cancer cell lines was treated with increasing concentrations of the AKT inhibitor GDC-0068 or the PI3K inhibitor GDC-0941. Pre- and posttreatment expression of EGFR and HER3 was compared using Western blot and correlated to probe accumulation with binding studies. Nude mice xenografts of HCC-70 or MDA-MB-468 were treated with either AKT inhibitor or PI3K inhibitor and imaged with either EGFR or HER3 PET probe.
Results: Changes in HER3 and EGFR PET probe accumulation correlate to RTK expression change as assessed by Western blot (R(2) of 0.85-0.98). EGFR PET probe PET/CT imaging of HCC70 tumors shows an SUV of 0.32 ± 0.03 for vehicle-, 0.50 ± 0.01 for GDC-0941-, and 0.62 ± 0.01 for GDC-0068-treated tumors, respectively (P < 0.01 for both comparisons to vehicle). HER3 PET probe PET/CT imaging of MDAMB468 tumors shows an SUV of 0.35 ± 0.02 for vehicle- and 0.73 ± 0.05 for GDC-0068-treated tumors (P < 0.01).
Conclusion: Our imaging studies, using PET probes specific to EGFR and HER3, show that changes in RTK expression indicative of resistance to PI3K and AKT inhibitors can be seen within days of therapy initiation and are of sufficient magnitude as to allow reliable clinical interpretation. Noninvasive PET monitoring of these RTK feedback loops should help to rapidly assess resistance to PI3K and AKT inhibitors and guide selection of an appropriate combinatorial therapeutic regimen on an individual patient basis.
Keywords: AKT; PI3K; breast cancer; imaging; receptor tyrosine kinase.
© 2016 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
Figures






References
-
- Zinda MJ, Johnson MA, Paul JD, et al. AKT-1, -2, and -3 are expressed in both normal and tumor tissues of the lung, breast, prostate, and colon. Clin Cancer Res. 2001;7:2475–2479. - PubMed
-
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
