Corneal endothelial cells possess an elaborate multipolar shape to maximize the basolateral to apical membrane area
- PMID: 27081293
- PMCID: PMC4814271
Corneal endothelial cells possess an elaborate multipolar shape to maximize the basolateral to apical membrane area
Abstract
Purpose: The corneal endothelium is widely believed to consist of geometrically regular cells interconnected by junctional complexes. However, while en face visualization of the endothelial apical surface reveals characteristic polygonal borders, the overall form of the component cells has rarely been observed.
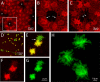
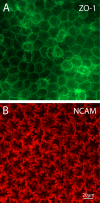
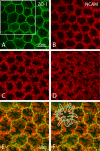
Methods: To visualize the shape of individual endothelial cells within the native monolayer, two independent Cre/LoxP-based cell labeling approaches were used. In the first, a P0-Cre mouse driver strain was bred to an R26-tdTomato reporter line to map neural crest-derived endothelial cells with cytosolic red fluorescent protein. In the second, HPRT-Cre induction of small numbers of green and red fluorescent protein-filled cells within a background of unlabeled cells was achieved using a dual-color reporter system, mosaic analysis with double markers (MADM). Selective imaging of the endothelial lateral membranes at different apicobasal levels was accomplished after staining with antibodies to ZO-1 and the neural cell adhesion molecule (NCAM).
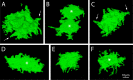
Results: When viewed in their entirety in whole-mount preparations, fluorescent protein-filled cells appear star-shaped, extending multiple dendritic processes that radiate outward in the plane of the monolayer. Examination of rare cases where cells expressing different fluorescent proteins lie directly adjacent to one another reveals that these long processes undergo extensive interdigitation. The resulting overlap allows individual cells to extend over a greater area than if the cell boundaries were mutually exclusive. Anti-NCAM staining of these interlocking peripheral cell extensions reveals an elaborate system of lateral membrane folds that, when viewed in optical sections, increase in complexity from the apical to the basal pole. This not only produces a substantial increase in the basolateral, relative to the apical, membrane but also greatly extends the paracellular pathway as a highly convoluted space.
Conclusions: Our analysis indicates that, far from being simple polygonal prisms, endothelial cells possess an elaborate multipolar shape. Their unusual geometry may be essential for the endothelium to carry out its role as the principal regulator of corneal extracellular fluid flux, and thus ultimately of tissue clarity.
Figures
References
-
- Mergler S, Pleyer U. The human corneal endothelium: new insights into electrophysiology and ion channels. Prog Retin Eye Res. 2007;26:359–78. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&lis... - PubMed
-
- Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res. 2012;95:2–7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&lis... - PMC - PubMed
-
- Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&lis... - PMC - PubMed
-
- Spring KR. Epithelial fluid transport – a century of investigation. News Physiol Sci. 1999;14:92–8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&lis... - PubMed
-
- Yamauchi Y, Abe K, Mantani A, Hitoshi Y, Suzuki M, Osuzu F, Kuratani S, Yamamura K. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev Biol. 1999;212:191–203. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&lis... - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

