The epigenetic modifier JMJD6 is amplified in mammary tumors and cooperates with c-Myc to enhance cellular transformation, tumor progression, and metastasis
- PMID: 27081402
- PMCID: PMC4831179
- DOI: 10.1186/s13148-016-0205-6
The epigenetic modifier JMJD6 is amplified in mammary tumors and cooperates with c-Myc to enhance cellular transformation, tumor progression, and metastasis
Abstract
Background: Oncogene overexpression in primary cells often triggers the induction of a cellular safeguard response promoting senescence or apoptosis. Secondary cooperating genetic events are generally required for oncogene-induced tumorigenesis to overcome these biologic obstacles. We employed comparative genomic hybridization for eight genetically engineered mouse models of mammary cancer to identify loci that might harbor genes that enhance oncogene-induced tumorigenesis.
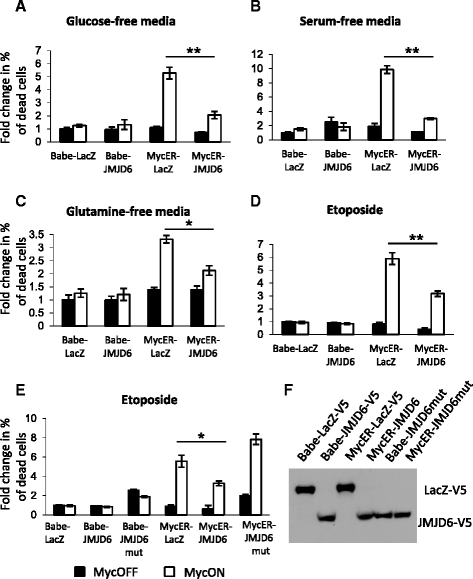
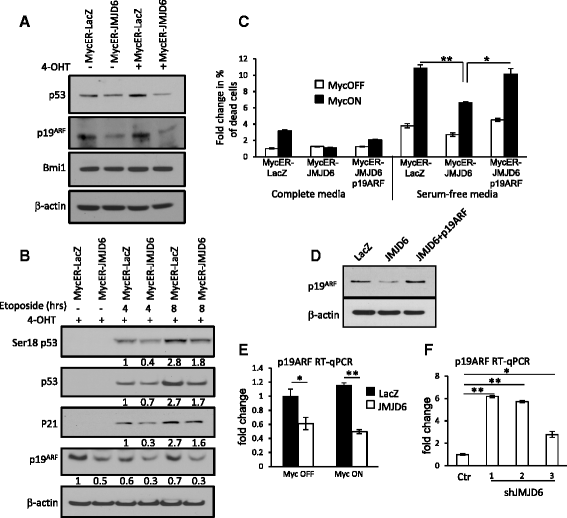
Results: Unlike many other mammary tumor models, the MMTV-Myc tumors displayed few copy number variants except for amplification of distal mouse chromosome 11 in 80 % of the tumors (syntenic to human 17q23-qter often amplified in human breast cancer). Analyses of candidate genes located in this region identified JMJD6 as an epigenetic regulatory gene that cooperates with Myc to enhance tumorigenesis. It suppresses Myc-induced apoptosis under varying stress conditions through inhibition of p19ARF messenger RNA (mRNA) and protein, leading to reduced levels of p53. JMJD6 binds to the p19ARF promoter and exerts its inhibitory function through demethylation of H4R3me2a. JMJD6 overexpression in MMTV-Myc cell lines increases tumor burden, induces EMT, and greatly enhances tumor metastasis. Importantly, we demonstrate that co-expression of high levels of JMJD6 and Myc is associated with poor prognosis for human ER+ breast cancer patients.
Conclusions: A novel epigenetic mechanism has been identified for how JMJD6 cooperates with Myc during oncogenic transformation. Combined high expression of Myc and JMJD6 confers a more aggressive phenotype in mouse and human tumors. Given the pleiotropic pro-tumorigenic activities of JMJD6, it may be useful as a prognostic factor and a therapeutic target for Myc-driven mammary tumorigenesis.
Keywords: Copy number variants; Epigenetics; JMJD6; Mammary cancer; Myc; Tumor progression.
Figures








References
-
- Green JE, Shibata MA, Yoshidome K, Liu ML, Jorcyk C, Anver MR, Wigginton J, Wiltrout R, Shibata E, Kaczmarczyk S, et al. The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene. 2000;19:1020–7. doi: 10.1038/sj.onc.1203280. - DOI - PubMed
-
- Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

