Natural history of LGMD2A for delineating outcome measures in clinical trials
- PMID: 27081656
- PMCID: PMC4818744
- DOI: 10.1002/acn3.287
Natural history of LGMD2A for delineating outcome measures in clinical trials
Abstract
Objective: Limb-girdle muscular dystophy 2A (LGMD2A, OMIM) is a slowly progressive myopathy caused by the deficiency in calpain 3, a calcium-dependent cysteine protease of the skeletal muscle.
Methods: In this study, we carried out an observational study of clinical manifestations and disease progression in genetically confirmed LGMD2A patients for up to 4 years. A total of 85 patients, aged 14-65 years, were recruited in three centers located in metropolitan France, the Basque country, and the Reunion Island. They were followed up every 6 months for 2 years and a subgroup was assessed annually thereafter for two more years. Data collected for all patients included clinical history, blood parameters, muscle strength assessed by manual muscle testing (MMT) and quantitative muscle testing, functional scores, and pulmonary and cardiac functions. In addition, CT scans of the lower limbs were performed in a subgroup of patients.
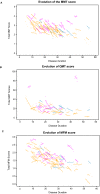
Results: Our study confirms the clinical description of a slowly progressive disorder with onset in the first or second decade of life with some degree of variability related to gender and mutation type. The null mutations lead to a more severe phenotype while compound heterozygote patients are the least affected. Muscle weakness is remarkably symmetrical and predominant in the axial muscles of the trunk and proximal muscles of the lower limb. There was a high correlation between the weakness at individual muscle level as assessed by MMT and the loss of density in CT scan analysis.
Interpretation: All the generated data will help to determine the endpoints for further clinical studies.
Figures







References
-
- Allamand V, Broux O, Bourg N, et al. Genetic heterogeneity of autosomal recessive limb‐girdle muscular dystrophy in a genetic isolate (Amish) and evidence for a new locus. Hum Mol Genet 1995;4(3):459–463. - PubMed
-
- Fardeau M, Hillaire D, Mignard C, et al. Juvenile limb‐girdle muscular dystrophy. Clinical, histopathological and genetic data from a small community living in the Reunion Island. Brain 1996;119(Pt 1):295–308. - PubMed
-
- Urtasun M, Saenz A, Roudaut C, et al. Limb‐girdle muscular dystrophy in Guipuzcoa (Basque Country, Spain). Brain 1998;121(Pt 9):1735–1747. - PubMed
-
- Beckmann JS, Richard I, Hillaire D, et al. A gene for limb‐girdle muscular dystrophy maps to chromosome 15 by linkage. C R Acad Sci III 1991;312(4):141–148. - PubMed
-
- Richard I, Broux O, Allamand V, et al. Mutations in the proteolytic enzyme calpain 3 cause limb‐girdle muscular dystrophy type 2A. Cell 1995;81(1):27–40. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

