A randomized placebo-controlled lovastatin trial for neurobehavioral function in neurofibromatosis I
- PMID: 27081657
- PMCID: PMC4818747
- DOI: 10.1002/acn3.288
A randomized placebo-controlled lovastatin trial for neurobehavioral function in neurofibromatosis I
Abstract
Objective: Lovastatin has been shown to reverse learning deficits in a mouse model of Neurofibromatosis Type 1 (NF1), a common monogenic disorder caused by a mutation in the Ras-MAPK pathway and associated with learning disabilities. We conducted a randomized double-blind placebo-controlled trial to assess lovastatin's effects on cognition and behavior in patients with NF1.
Method: Forty-four NF1 patients (mean age 25.7+/-11.6 years; 64% female) were randomly assigned to 14 weeks of lovastatin (N = 23; maximum dose of 80 mg/day for adult participants and 40 mg/day for children) or placebo (N = 21). Based on findings in the mouse model, primary outcome measures were nonverbal learning and working memory. Secondary outcome measures included verbal memory, attention, and self/parent-reported behavioral problems, as well as tolerability of medication. Participants also underwent neuroimaging assessments at baseline and 14 weeks, to determine whether neural biomarkers were associated with treatment response. Linear mixed models assessed for differential treatment effects on outcome measures.
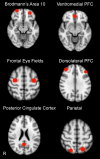
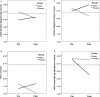
Results: Twelve participants dropped from the study prior to completion (8 placebo, 4 lovastatin), resulting in 32 completers (15 placebo, 17 lovastatin). Lovastatin was well-tolerated, with no serious adverse events. Differential improvement favoring lovastatin treatment was observed for one primary (working memory; effect size f (2) = 0.70, P < 0.01) and two secondary outcome measures (verbal memory, f (2) = 0.19, P = 0.02, and adult self-reported internalizing problems, f (2) = 0.26, P = 0.03). Exploratory moderator analyses revealed that higher baseline neural activity in frontal regions was associated with larger treatment effects.
Interpretation: These preliminary results suggest beneficial effects of lovastatin on some learning and memory functions, as well as internalizing symptoms in patients with NF1.
Figures
Similar articles
-
Statins in Children with Neurofibromatosis Type 1: A Systematic Review of Randomized Controlled Trials.Children (Basel). 2023 Sep 15;10(9):1556. doi: 10.3390/children10091556. Children (Basel). 2023. PMID: 37761518 Free PMC article. Review.
-
Randomized placebo-controlled study of lovastatin in children with neurofibromatosis type 1.Neurology. 2016 Dec 13;87(24):2575-2584. doi: 10.1212/WNL.0000000000003435. Epub 2016 Nov 9. Neurology. 2016. PMID: 27956565 Free PMC article. Clinical Trial.
-
Simvastatin for cognitive deficits and behavioural problems in patients with neurofibromatosis type 1 (NF1-SIMCODA): a randomised, placebo-controlled trial.Lancet Neurol. 2013 Nov;12(11):1076-83. doi: 10.1016/S1474-4422(13)70227-8. Epub 2013 Oct 1. Lancet Neurol. 2013. PMID: 24090588 Clinical Trial.
-
Lovastatin improves impaired synaptic plasticity and phasic alertness in patients with neurofibromatosis type 1.BMC Neurol. 2013 Oct 2;13:131. doi: 10.1186/1471-2377-13-131. BMC Neurol. 2013. PMID: 24088225 Free PMC article. Clinical Trial.
-
Cognitive and psychomotor effects of risperidone in schizophrenia and schizoaffective disorder.Clin Ther. 2008 Sep;30(9):1565-89. doi: 10.1016/j.clinthera.2008.09.014. Clin Ther. 2008. PMID: 18840365 Review.
Cited by
-
Molecules linked to Ras signaling as therapeutic targets in cardiac pathologies.Biol Res. 2021 Aug 3;54(1):23. doi: 10.1186/s40659-021-00342-6. Biol Res. 2021. PMID: 34344467 Free PMC article. Review.
-
Spatial working memory in neurofibromatosis 1: Altered neural activity and functional connectivity.Neuroimage Clin. 2017 Jun 27;15:801-811. doi: 10.1016/j.nicl.2017.06.032. eCollection 2017. Neuroimage Clin. 2017. PMID: 28725547 Free PMC article.
-
Mechanistic insights from animal models of neurofibromatosis type 1 cognitive impairment.Dis Model Mech. 2022 Aug 1;15(8):dmm049422. doi: 10.1242/dmm.049422. Epub 2022 Aug 29. Dis Model Mech. 2022. PMID: 36037004 Free PMC article. Review.
-
Statins in Children with Neurofibromatosis Type 1: A Systematic Review of Randomized Controlled Trials.Children (Basel). 2023 Sep 15;10(9):1556. doi: 10.3390/children10091556. Children (Basel). 2023. PMID: 37761518 Free PMC article. Review.
-
Genetic and Environmental Contributions to Autism Spectrum Disorder Through Mechanistic Target of Rapamycin.Biol Psychiatry Glob Open Sci. 2021 Sep 1;2(2):95-105. doi: 10.1016/j.bpsgos.2021.08.005. eCollection 2022 Apr. Biol Psychiatry Glob Open Sci. 2021. PMID: 36325164 Free PMC article. Review.
References
-
- Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor‐prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A 2010;152A:327–332. - PubMed
-
- Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 2005;65:1037–1044. - PubMed
-
- North KN, Riccardi V, Samango‐Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis. 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology 1997;48:1121–1127. - PubMed
-
- Mautner VF, Granstrom S, Leark RA. Impact of ADHD in adults with neurofibromatosis type 1: associated psychological and social problems. J Attention Disord 2015;19:35–43. - PubMed
-
- Garg S, Lehtonen A, Huson SM, et al. Autism and other psychiatric comorbidity in neurofibromatosis type 1: evidence from a population‐based study. Dev Med Child Neurol 2013;55:139–145. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

