Decreased Polycystin 2 Levels Result in Non-Renal Cardiac Dysfunction with Aging
- PMID: 27081851
- PMCID: PMC4833351
- DOI: 10.1371/journal.pone.0153632
Decreased Polycystin 2 Levels Result in Non-Renal Cardiac Dysfunction with Aging
Abstract
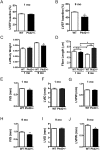
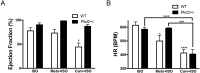
Mutations in the gene for polycystin 2 (Pkd2) lead to polycystic kidney disease, however the main cause of mortality in humans is cardiac related. We previously showed that 5 month old Pkd2+/- mice have altered calcium-contractile activity in cardiomyocytes, but have preserved cardiac function. Here, we examined 1 and 9 month old Pkd2+/- mice to determine if decreased amounts of functional polycystin 2 leads to impaired cardiac function with aging. We observed changes in calcium handling proteins in 1 month old Pkd2+/- mice, and these changes were exacerbated in 9 month old Pkd2+/- mice. Anatomically, the 9 month old Pkd2+/- mice had thinner left ventricular walls, consistent with dilated cardiomyopathy, and the left ventricular ejection fraction was decreased. Intriguingly, in response to acute isoproterenol stimulation to examine β-adrenergic responses, the 9 month old Pkd2+/- mice exhibited a stronger contractile response, which also coincided with preserved localization of the β2 adrenergic receptor. Importantly, the Pkd2+/- mice did not have any renal impairment. We conclude that the cardiac-related impact of decreased polycystin 2 progresses over time towards cardiac dysfunction and altered adrenergic signaling. These results provide further evidence that polycystin 2 provides a critical function in the heart, independent of renal involvement.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

