Alemtuzumab for multiple sclerosis
- PMID: 27082500
- PMCID: PMC6486037
- DOI: 10.1002/14651858.CD011203.pub2
Alemtuzumab for multiple sclerosis
Update in
-
Alemtuzumab for multiple sclerosis.Cochrane Database Syst Rev. 2023 Jun 5;6(6):CD011203. doi: 10.1002/14651858.CD011203.pub3. Cochrane Database Syst Rev. 2023. PMID: 37272540 Free PMC article. Review.
Abstract
Background: Multiple sclerosis (MS) is an autoimmune, T-cell-dependent, inflammatory, demyelinating disease of the central nervous system, with an unpredictable course. Current MS therapies focus on treating exacerbations, preventing new exacerbations and avoiding the progression of disability. However, at present there is no effective treatment that is capable of safely and effectively reaching these objectives. This has led to the development and investigation of new drugs. Recent clinical trials suggest that alemtuzumab, a humanised monoclonal antibody against cell surface CD52, could be a promising option for MS.
Objectives: To assess the safety and effectiveness of alemtuzumab used alone or associated with other treatments to decrease disease activity in patients with any form of MS.
Search methods: We searched the Trials Register of the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group (30 April 2015), which contains trials from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, LILACS and the trial registry databases ClinicalTrials.gov and WHO International Clinical Trials Registry Platform. There was no restriction on the source, publication date or language.
Selection criteria: All randomised clinical trials (RCTs) involving adults diagnosed with any form of MS according to the McDonald criteria, comparing alemtuzumab alone or associated with other medications, at any dose and for any duration, versus placebo or any other active drug therapy or alemtuzumab in other dose, regimen or duration. The co-primary outcomes were relapse-free survival, sustained disease progression and number of participants with at least one of any adverse events, including serious adverse events.
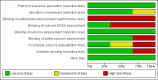
Data collection and analysis: Two independent review authors performed study selection, data extraction and 'Risk of bias' assessment. A third review author checked the process for accuracy. We used the Cochrane 'Risk of bias' tool to assess the risk of bias of the studies included in the review. We used the GRADE system to assess the quality of the body of evidence. To measure the treatment effect on dichotomous outcomes we used the risk ratio (RR); for the treatment effect on continuous outcomes, we used the mean difference (MD) and for time-to-event outcomes we used hazard ratio (HR). We calculated 95% confidence intervals (CI) for these measures. When there was no heterogeneity, we used a fixed-effect model to pool data.
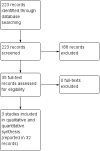
Main results: Three RCTs (1713 participants) fulfilled the selection criteria and we included them in the review. All three trials compared alemtuzumab versus subcutaneous interferon beta-1a for patients with relapsing-remitting MS. Patients were treatment-naive in the CARE-MS and CAMMS223 studies. The CARE-MS II study included patients with at least one relapse while being treated with interferon beta or glatiramer acetate. Alemtuzumab was given for 12 or 24 months; for some outcomes, the follow-up period reached 36 months. The regimens were (a) 12 mg or 24 mg per day administered intravenously, once a day for five consecutive days at month 0 and 12 or (b) 24 mg per day, intravenously, once a day for three consecutive days at month 12 and 24. The patients in the other arm of the trials received interferon beta-1a 44 μg subcutaneously three times weekly after dose titration.At 24 months, alemtuzumab 12 mg was associated with: (a) higher relapse-free survival (hazard ratio (HR) 0.50, 95% CI 0.41 to 0.60; 1248 participants, two studies, moderate quality evidence); (b) higher sustained disease progression-free survival (HR 0.62, 95% CI 0.44 to 0.87; 1191 participants; two studies; moderate quality evidence); (c) a slightly higher number of participants with at least one adverse event (RR 1.04, 95% CI 1.01 to 1.06; 1248 participants; two studies; moderate quality evidence); (d) a lower number of participants with new or enlarging T2-hyperintense lesions on magnetic resonance imaging (MRI) (RR 0.74, 95% CI 0.59 to 0.91; 1238 participants; two studies; I(2) = 80%); and (e) a lower number of dropouts (RR 0.31, 95% CI 0.23 to 0.41; 1248 participants; two studies, I(2) = 29%; low quality evidence).At 36 months, alemtuzumab 24 mg was associated with: (a) higher relapse-free survival (45 versus 17; HR 0.21, 95% CI 0.11 to 0.40; one study; 221 participants); (b) a higher sustained disease progression-free survival (HR 0.33, 95% CI 0.16 to 0.69; one study; 221 participants); and (c) no statistical difference in the rate of participants with at least one adverse event. We did not find any study that reported any of the following outcomes: rate of participants free of clinical disease activity, quality of life, fatigue or change in the numbers of MRI T2- and T1-weighted lesions after treatment. It was not possible to perform subgroup analyses according to disease type and disability at baseline due to lack of data.
Authors' conclusions: In patients with relapsing-remitting MS, alemtuzumab 12 mg was better than subcutaneous interferon beta-1a for the following outcomes assessed at 24 months: relapse-free survival, sustained disease progression-free survival, number of participants with at least one adverse event and number of participants with new or enlarging T2-hyperintense lesions on MRI. The quality of the evidence for these results was low to moderate. Alemtuzumab 24 mg seemed to be better than subcutaneous interferon beta-1a for relapse-free survival and sustained disease progression-free survival, at 36 months.More randomised clinical trials are needed to evaluate the effects of alemtuzumab on other forms of MS and compared with other therapeutic options. These new studies should assess additional relevant outcomes such as the rate of participants free of clinical disease activity, quality of life, fatigue and adverse events (individual rates, serious adverse events and long-term adverse events). Moreover, these new studies should evaluate other doses and durations of alemtuzumab course.
Conflict of interest statement
RR: none
GJMP: none
MRT: none
Figures










References
References to studies included in this review
CAMMS223 {published data only}
-
- Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, et al. Alemtuzumab vs. interferon beta‐1a in early multiple sclerosis. New England Journal of Medicine 2008;359(17):1786‐801. - PubMed
-
- Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, et al. Alemtuzumab more effective than interferon β‐1a at 5‐year follow‐up of CAMMS223 clinical trial. Neurology 2012;78:1069–78. - PubMed
-
- Coles AJ, Fox E, Vladic A, Gazda SK, Brinar V, Selmaj KW, et al. Alemtuzumab versus interferon beta‐1a in early relapsing‐remitting multiple sclerosis: post‐hoc and subset analyses of clinical efficacy outcomes. Lancet Neurology 2011;10(4):338‐48. - PubMed
-
- Daniels GH, Vladic A, Brinar V, Zavalishin I, Valente W, Oyuela P, et al. Alemtuzumab‐related thyroid dysfunction in a phase 2 trial of patients with relapsing‐remitting multiple sclerosis. Journal of Clinical Endocrinology and Metabolism 2014;99(1):80‐9. - PubMed
CARE‐MS I {published data only}
-
- Arnold D, Brinar V, Cohen J, Coles A, Confavreux C, Fisher E, et al. Effect of alemtuzumab vs. RebifTM on brain MRI measurements: results of CARE‐MS I, a phase 3 study. Neurology 2012;78(1):S11.006.
-
- Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first‐line treatment for patients with relapsing‐remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380(9856):1819‐28. - PubMed
-
- Coles A, Brinar V, Arnold D, Cohen J, Confavreux C, Fox E, et al. Efficacy and safety results from comparison of alemtuzumab and RebifTM efficacy in multiple sclerosis I (CARE‐MS I): a phase 3 study in relapsing‐remitting treatment‐naive patients. Neurology 2012;78(Suppl 1):S01.006.
-
- Fox E, Arnold D, Brinar V, Cohen J, Coles A, Confavreux C, et al. Relapse outcomes with alemtuzumab vs. RebifTM in treatment‐naive relapsing‐remitting multiple sclerosis (CARE‐MS I): secondary and tertiary endpoints. Neurology 2012;78(Suppl 1):PD5.004. [DOI: 10.1212/WNL.78.1_MeetingAbstracts.PD5.004] - DOI
-
- Giovannoni G, Arnold DL, Cohen J, Coles AJ, Confavreux C, Fox HP, et al. Disease activity‐free status in comparison of alemtuzumab and RebifTM efficacy in multiple sclerosis I (CARE‐MS I) phase 3 study. Journal of Neurology 2012;259(Suppl 1):47.
CARE‐MS II {published data only}
-
- Arnold DL, Cohen J, Coles AJ, Confavreux C, Fisher E, Fox EJ, et al. Effect of alemtuzumab vs. Rebif® on brain MRI measurements. Multiple Sclerosis 2012;18(4):397.
-
- Arroyo R, Arnold DL, Cohen JA, Coles AJ, Confavreux C, Fox EJ, et al. Alemtuzumab improves quality of life compared to SC IFNB‐1a in CARE‐MS II. Journal of Neurology 2013;260:S121‐2.
-
- Barkhof F, Fisher E, Palmer J, Margolin DH, Arnold DL. Alemtuzumab demonstrates improvement in MRI outcomes across baseline subgroups versus subcutaneous interferon beta‐1a in relapsing‐remitting multiple sclerosis patients who relapsed on prior therapy. European Journal of Neurology 2014;21:126‐7.
-
- Brinar V, Arnold DL, Cohen J, Coles AJ, Fox EJ, Hartung HP, et al. Alemtuzumab improves expanded disability status scale (EDSS) via effects on functional systems: CARE‐MS II. Multiple Sclerosis. Multiple Sclerosis Journal 2013;19(S1):283‐4.
-
- Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease‐modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829‐39. - PubMed
Additional references
CADTH 2013
-
- Canadian Agency for Drugs and Technologies in Health. Management of relapsing‐remitting multiple sclerosis. http://www.ncbi.nlm.nih.gov/books/NBK169748/ (accessed 11 March 2016).
Coles 1999a
-
- Coles AJ, Wing M, Smith S, Coraddu F, Greer S, Taylor C, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 1999;354:1691‐5. - PubMed
Coles 1999b
-
- Coles AJ, Wing MG, Molyneux P, Paolillo A, Davie CM, Hale G, et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Annals of Neurology 1999;46:296‐304. - PubMed
Coles 2006
-
- Coles AJ, Cox A, Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. Journal of Neurology 2006;253:98‐108. - PubMed
Cossburn 2011
-
- Cossburn M, Pace AA, Jones J, Ali R, Ingram G, Baker K, et al. Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology 2011;77(6):573‐9. - PubMed
EMA 2013
-
- European Medicines Agency. Lemtrada ‐ Committee for Medicinal Products for Human Use (CHMP) ‐ Assessment report. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_asses... (accessed 11 March 2016).
FDA 2001
-
- US Food, Drug Administration. Campath (alemtuzumab) Product Approval Information ‐ Application number BLA 103948/0. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/103948_0000_Campa... (accessed 11 March 2016).
FDA 2014
-
- US Food, Drug Administration. Alemtuzumab (Lemtrada) Product Approval Information. Licensing Action 2014. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/103948Orig1... (accessed 11 March 2016).
FDA 2015
-
- US Food, Drug Administration. Risk Evaluation and Mitigation Strategies (REMS). Lemtrada 2015. http://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRems... (accessed 11 March 2016).
Filippini 2013
Fischer 1999
-
- Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Multiple Sclerosis 1999;5:251‐9. - PubMed
Genzyme 2013
-
- Peripheral and Central Nervous System Drugs Advisory Committee. Alemtuzumab Advisory Committee Briefing Document. BLA 103948. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMateria... (accessed 11 March 2016).
Gilleece 1993
-
- Gilleece M, Dexter T. Effect of campath‐1h antibody on human hematopoietic progenitors in vitro. Blood 1993;82:807–12. - PubMed
Gomez‐Almaguer 2012
-
- Gomez‐Almaguer D, Jaime‐Perez J, Ruiz‐Arguelles G. Antibodies in the treatment of aplastic anaemia. Archivum Immunologiae et Therapiae Experimentalis 2012;60:99‐106. - PubMed
GRADEpro 2008 [Computer program]
-
- GRADE Working Group. GRADEpro. Version 3.2 for Windows. GRADE Working Group, 2008.
Gray 2004
Hawkins 1999
Healy 2013
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hill‐Cawthorne 2012
-
- Hill‐Cawthorne GA, Button T, Tuohy O, Jones JL, May K, Somerfield J, et al. Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry 2012;83(3):298‐304. - PubMed
Hirst 2008
-
- Hirst CL, Pace A, Pickersgill TP, Jones R, McLean BN, Zajicek JP, et al. Campath1‐H treatment in patients with aggressive relapsing remitting multiple sclerosis. Journal of Neurology 2008;255:231‐8. - PubMed
Keating 2002
-
- Keating M, Flinn I, Jain V, Binet J, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath‐1H) in patients who have failed fludarabine: results of a large international study. Blood 2002;99:3554–61. - PubMed
Krupp 1989
-
- Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 1989;46:1121–3. - PubMed
Kurtzke 1983
-
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale. Neurology 1983;33:1444‐52. - PubMed
Li 1999
-
- Li DK, Paty DW, the UBC MS/MRI Analysis Research Group and the PRISMS Study Group. Magnetic resonance imaging results of the PRISMS trial: a randomised, double‐blind, placebo‐controlled study of interferon‐beta la in relapsing–remitting multiple sclerosis. Annals of Neurology 1999;46:197‐206. - PubMed
Lockwood 2003
-
- Lockwood C, Hale G, Waldmann H, Jayne D. Remission induction in Behcet’s disease following lymphocyte depletion by the anti‐CD52 antibody CAMPATH‐1H. Rheumatology 2003;42:1539‐44. - PubMed
Lublin 1996
-
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology 1996;46:907‐11. - PubMed
McDonald 2001
-
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Annals of Neurology 2001;50:121‐7. - PubMed
Multiple Sclerosis International Federation 2010
-
- Multiple Sclerosis International Federation (MSIF). Global Economic Impact of Multiple Sclerosis. http://www.msif.org/wp‐content/uploads/2014/09/Global_economic_impact_of... (accessed 11 March 2016).
Noseworthy 2000
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. New England Journal of Medicine 2000;343(13):938‐52. - PubMed
Polman 2011
Poser 1983
-
- Poser CM, Paty DW, Scheinberg L, McDonal WI, Davis FA, Ebers GC. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13:227‐31. - PubMed
Rao 2012
RevMan 2015 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Rieckmann 2009
-
- Rieckmann P. Concepts of induction and escalation therapy in multiple sclerosis. Journal of Neurosurgical Sciences 2009;277(Suppl 1):42‐5. - PubMed
Scolding 2015
-
- Scolding N, Barnes D, Cader S, Chataway J, Chaudhuri A, Coles A, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease‐modifying treatments in multiple sclerosis. Practical Neurology 2015;15(4):273‐9. - PubMed
Vickrey 1995
-
- Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health‐related quality of life measure for multiple sclerosis. Quality of Life Research 1995;4(3):187‐206. - PubMed
Waldmann 2005
Weissenbacher 2010
-
- Weissenbacher A, Boesmueller C, Brandacher G, Oellinger R, Pratschke J, Schneeberger S. Alemtuzumab in solid organ transplantation and in composite tissue allotransplantation. Immunotherapy 2010;2:783‐90. - PubMed
Xia 1991
-
- Xia M, Tone M, Packman L, Hale G, Waldmann H. Characterization of the Campath‐1 (CDW2) antigen: biochemical analysis and cDNA cloning reveal an unusually small peptide backbone. European Journal of Immunology 1991;21:1677‐84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

