Neuroprotective Role of L-NG-Nitroarginine Methyl Ester (L-NAME) against Chronic Hypobaric Hypoxia with Crowding Stress (CHC) Induced Depression-Like Behaviour
- PMID: 27082990
- PMCID: PMC4833384
- DOI: 10.1371/journal.pone.0153371
Neuroprotective Role of L-NG-Nitroarginine Methyl Ester (L-NAME) against Chronic Hypobaric Hypoxia with Crowding Stress (CHC) Induced Depression-Like Behaviour
Abstract
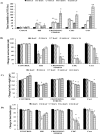
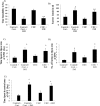
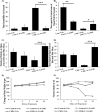
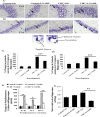
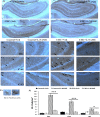
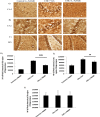
Improper neuroimmune responses following chronic stress exposure have been reported to cause neuronal dysfunctions leading to memory impairment, anxiety and depression like behaviours. Though several factors affecting microglial activation and consequent alteration in neuro-inflammatory responses have been well studied, role of NO and its association with microglia in stress induced depression model is yet to be explored. In the present study, we validated combination of chronic hypobaric hypoxia and crowding (CHC) as a stress model for depression and investigated the role of chronic stress induced elevated nitric oxide (NO) level in microglia activation and its effect on neuro-inflammatory responses in brain. Further, we evaluated the ameliorative effect of L-NG-Nitroarginine Methyl Ester (L-NAME) to reverse the stress induced depressive mood state. Four groups of male Sprague Dawley rat were taken and divided into control and CHC stress exposed group with and without treatment of L-NAME. Depression like behaviour and anhedonia in rats were assessed by Forced Swim Test (FST) and Sucrose Preference Test (SPT). Microglial activation was evaluated using Iba-1 immunohistochemistry and proinflammatory cytokines were assessed in the hippocampal region. Our result showed that exposure to CHC stress increased the number of active microglia with corresponding increase in inflammatory cytokines and altered behavioural responses. The inhibition of NO synthesis by L-NAME during CHC exposure decreased the number of active microglia in hippocampus as evident from decreased Iba-1 positive cells. Further, L-NAME administration decreased pro-inflammatory cytokines in hippocampus and improved behaviour of rats. Our study demonstrate that stress induced elevation of NO plays pivotal role in altered microglial activation and consequent neurodegenerative processes leading to depression like behaviour in rat.
Conflict of interest statement
Figures



References
-
- García-Bueno B, Madrigal JL, Pérez-Nievas BG, Leza JC (2008) Stress mediators regulate brain prostaglandin synthesis and peroxisome proliferator-activated receptor-gamma activation after stress in rats. Endocrinology 149: 1969–1978. - PubMed
-
- Tilleux S, Berger J, Hermans E (2007) Induction of astrogliosis by activated microglia is associated with a down-regulation of metabotropic glutamate receptor 5. J Neuroimmunol 189: 23–30. - PubMed
-
- Maes M, Bosmans E, Suy E, Vandervorst C, De Jonckheere C, Raus J (1990) Immune disturbances during major depression: upregulated expression of interleukin-2 receptors. Neuropsychobiology 24: 115–120 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

