Osteopontin protects against high phosphate-induced nephrocalcinosis and vascular calcification
- PMID: 27083280
- PMCID: PMC4834144
- DOI: 10.1016/j.kint.2015.12.046
Osteopontin protects against high phosphate-induced nephrocalcinosis and vascular calcification
Abstract
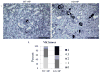
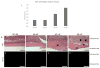
Pathologic calcification is a significant cause of increased morbidity and mortality in patients with chronic kidney disease. The precise mechanisms of ectopic calcification are not fully elucidated, but it is known to be caused by an imbalance of procalcific and anticalcific factors. In the chronic kidney disease population, an elevated phosphate burden is both highly prevalent and a known risk factor for ectopic calcification. Here we tested whether osteopontin, an inhibitor of calcification, protects against high phosphate load-induced nephrocalcinosis and vascular calcification. Osteopontin knockout mice were placed on a high phosphate diet for 11 weeks. Osteopontin deficiency together with phosphate overload caused uremia, nephrocalcinosis characterized by substantial renal tubular and interstitial calcium deposition, and marked vascular calcification when compared with control mice. Although the osteopontin-deficient mice did not exhibit hypercalcemia or hyperphosphatemia, they did show abnormalities in the mineral metabolism hormone fibroblast growth factor-23. Thus, endogenous osteopontin plays a critical role in the prevention of phosphate-induced nephrocalcinosis and vascular calcification in response to high phosphate load. A better understanding of osteopontin's role in phosphate-induced calcification will hopefully lead to better biomarkers and therapies for this disease, especially in patients with chronic kidney disease and other at-risk populations.
Keywords: calcium; fibroblast growth factor-23; parathyroid hormone; phosphate; uremia; vascular calcification.
Copyright © 2016 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors have no competing interests.
Figures




References
-
- Shavit L, Jaeger P, Unwin RJ. What is nephrocalcinosis? Kidney Int. 2015;88:35–43. - PubMed
-
- Witteman JC, Kok FJ, van Saase JL, et al. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–1122. - PubMed
-
- Witteman JC, Kannel WB, Wolf PA, et al. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am J Cardiol. 1990;66:1060–1064. - PubMed
-
- Adragao T, Pires A, Lucas C, et al. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1480–1488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

