Connexin 43 in astrocytes contributes to motor neuron toxicity in amyotrophic lateral sclerosis
- PMID: 27083773
- PMCID: PMC5635605
- DOI: 10.1002/glia.22989
Connexin 43 in astrocytes contributes to motor neuron toxicity in amyotrophic lateral sclerosis
Abstract
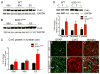
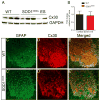
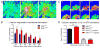
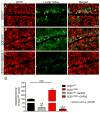
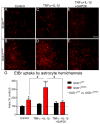
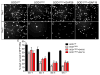
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by progressive loss of motor neurons in the CNS. Astrocytes play a critical role in disease progression of ALS. Astrocytes are interconnected through a family of gap junction proteins known as connexins (Cx). Cx43 is a major astrocyte connexin conducting crucial homeostatic functions in the CNS. Under pathological conditions, connexin expression and functions are altered. Here we report that an abnormal increase in Cx43 expression serves as one of the mechanisms for astrocyte-mediated toxicity in ALS. We observed a progressive increase in Cx43 expression in the SOD1(G93A) mouse model of ALS during the disease course. Notably, this increase in Cx43 was also detected in the motor cortex and spinal cord of ALS patients. Astrocytes isolated from SOD1(G93A) mice as well as human induced pluripotent stem cell (iPSC)-derived astrocytes showed an increase in Cx43 protein, which was found to be an endogenous phenomenon independent of neuronal co-culture. Increased Cx43 expression led to important functional consequences when tested in SOD1(G93A) astrocytes when compared to control astrocytes over-expressing wild-type SOD1 (SOD1(WT) ). We observed SOD1(G93A) astrocytes exhibited enhanced gap junction coupling, increased hemichannel-mediated activity, and elevated intracellular calcium levels. Finally, we tested the impact of increased expression of Cx43 on MN survival and observed that use of both a pan Cx43 blocker and Cx43 hemichannel blocker conferred neuroprotection to MNs cultured with SOD1(G93A) astrocytes. These novel findings show a previously unrecognized role of Cx43 in ALS-related motor neuron loss. GLIA 2016;64:1154-1169.
Keywords: ALS; astrocyte; connexin; iPSC; motor neuron.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
The authors do not have any conflicts of interest to declare.
Figures


References
-
- Abudara V, Roux L, Dallerac G, Matias I, Dulong J, Mothet JP, Rouach N, Giaume C. Activated microglia impairs neuroglial interaction by opening Cx43 hemichannels in hippocampal astrocytes. Glia. 2015;63:795–811. - PubMed
-
- Avendano BC, Montero TD, Chavez CE, von Bernhardi R, Orellana JA. Prenatal exposure to inflammatory conditions increases Cx43 and Panx1 unopposed channel opening and activation of astrocytes in the offspring effect on neuronal survival. Glia. 2015;63:2058–2072. - PubMed
-
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

