Vestibular compensation: the neuro-otologist's best friend
- PMID: 27083885
- PMCID: PMC4833803
- DOI: 10.1007/s00415-015-7903-4
Vestibular compensation: the neuro-otologist's best friend
Abstract
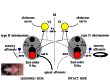
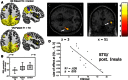
Why vestibular compensation (VC) after an acute unilateral vestibular loss is the neuro-otologist's best friend is the question at the heart of this paper. The different plasticity mechanisms underlying VC are first reviewed, and the authors present thereafter the dual concept of vestibulo-centric versus distributed learning processes to explain the compensation of deficits resulting from the static versus dynamic vestibular imbalance. The main challenges for the plastic events occurring in the vestibular nuclei (VN) during a post-lesion critical period are neural protection, structural reorganization and rebalance of VN activity on both sides. Data from animal models show that modulation of the ipsilesional VN activity by the contralateral drive substitutes for the normal push-pull mechanism. On the other hand, sensory and behavioural substitutions are the main mechanisms implicated in the recovery of the dynamic functions. These newly elaborated sensorimotor reorganizations are vicarious idiosyncratic strategies implicating the VN and multisensory brain regions. Imaging studies in unilateral vestibular loss patients show the implication of a large neuronal network (VN, commissural pathways, vestibulo-cerebellum, thalamus, temporoparietal cortex, hippocampus, somatosensory and visual cortical areas). Changes in gray matter volume in these multisensory brain regions are structural changes supporting the sensory substitution mechanisms of VC. Finally, the authors summarize the two ways to improve VC in humans (neuropharmacology and vestibular rehabilitation therapy), and they conclude that VC would follow a "top-down" strategy in patients with acute vestibular lesions. Future challenges to understand VC are proposed.
Keywords: Animal models; Dynamic deficits recovery; Human brain imaging; Static deficits recovery; Unilateral vestibular loss; Vestibular compensation.
Figures


References
-
- Lacour M, Borel L. Vestibular control of posture and gait. Arch Ital Biol. 1993;131:81–104. - PubMed
-
- Wilson VJ, Melvill Jones GM. Mammalian vestibular physiology. New York, London: Plenum Press; 1979.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

