Managing leptomeningeal melanoma metastases in the era of immune and targeted therapy
- PMID: 27084046
- PMCID: PMC4939138
- DOI: 10.1002/ijc.30147
Managing leptomeningeal melanoma metastases in the era of immune and targeted therapy
Abstract
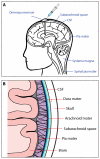
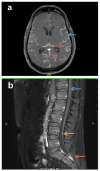
Melanoma frequently metastasizes to the brain, with CNS involvement being clinically evident in ∼30% of patients (as high as 75% at autopsy). In ∼5% cases melanoma cells also metastasize to the leptomeninges, the sub-arachnoid space and cerebrospinal fluid (CSF). Patients with leptomeningeal melanoma metastases (LMM) have the worst prognosis and are characterized by rapid disease progression (mean survival 8-10 weeks) and a death from neurological causes. The recent years have seen tremendous progress in the development of targeted and immune therapies for melanoma that has translated into an increased survival benefit. Despite these gains, the majority of patients fail therapy and there is a suspicion that the brain and the leptomeninges are a "sanctuary" sites for melanoma cells that escape both targeted therapy and immunologic therapies. Emerging evidence suggests that (1) Cancer cells migrating to the CNS may have unique molecular properties and (2) the CNS/leptomeningeal microenvironment represents a pro-survival niche that influences therapeutic response. In this Mini-Review, we will outline the clinical course of LMM development and will describe how the intracranial immune and cellular microenvironments offer both opportunities and challenges for the successful management of this disease. We will further discuss the latest data demonstrating the potential use of BRAF inhibitors and immune therapy in the management of LMM, and will review future potential therapeutic strategies for the management of this most devastating complication of advanced melanoma.
Keywords: BRAF; brain; immunotherapy; leptomeninges; melanoma.
© 2016 UICC.
Figures
References
-
- Kenchappa RS, Tran N, Rao NG, Smalley KS, Gibney GT, Sondak VK, Forsyth PA. Novel Treatments for Melanoma Brain Metastases. Cancer Control. 2013;20:298–306. - PubMed
-
- Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, Hwu P, Bedikian A. Prognostic Factors for Survival in Melanoma Patients With Brain Metastases. Cancer. 2011;117:1687–96. - PubMed
-
- Fife KM, Colman MH, Stevens GN, Firth IC, Moon D, Shannon KF, Harman R, Petersen-Schaefer K, Zacest AC, Besser M, Milton GW, McCarthy WH, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22:1293–300. - PubMed
-
- Carlino MS, Fogarty GB, Long GV. Treatment of melanoma brain metastases: a new paradigm. Cancer J. 2012;18:208–12. - PubMed
-
- Agarwala SS, Kirkwood JM, Gore M, Dreno B, Thatcher N, Czarnetski B, Atkins M, Buzaid A, Skarlos D, Rankin EM. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22:2101–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

