Monoaminergic neuropathology in Alzheimer's disease
- PMID: 27084356
- PMCID: PMC5061605
- DOI: 10.1016/j.pneurobio.2016.04.001
Monoaminergic neuropathology in Alzheimer's disease
Abstract
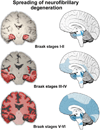
None of the proposed mechanisms of Alzheimer's disease (AD) fully explains the distribution patterns of the neuropathological changes at the cellular and regional levels, and their clinical correlates. One aspect of this problem lies in the complex genetic, epigenetic, and environmental landscape of AD: early-onset AD is often familial with autosomal dominant inheritance, while the vast majority of AD cases are late-onset, with the ε4 variant of the gene encoding apolipoprotein E (APOE) known to confer a 5-20 fold increased risk with partial penetrance. Mechanisms by which genetic variants and environmental factors influence the development of AD pathological changes, especially neurofibrillary degeneration, are not yet known. Here we review current knowledge of the involvement of the monoaminergic systems in AD. The changes in the serotonergic, noradrenergic, dopaminergic, histaminergic, and melatonergic systems in AD are briefly described. We also summarize the possibilities for monoamine-based treatment in AD. Besides neuropathologic AD criteria that include the noradrenergic locus coeruleus (LC), special emphasis is given to the serotonergic dorsal raphe nucleus (DRN). Both of these brainstem nuclei are among the first to be affected by tau protein abnormalities in the course of sporadic AD, causing behavioral and cognitive symptoms of variable severity. The possibility that most of the tangle-bearing neurons of the LC and DRN may release amyloid β as well as soluble monomeric or oligomeric tau protein trans-synaptically by their diffuse projections to the cerebral cortex emphasizes their selective vulnerability and warrants further investigations of the monoaminergic systems in AD.
Keywords: 5-hydroxytryptamine (serotonin); Alzheimer’s disease; Amyloid beta (Aβ) peptide; Blood-brain barrier; Cerebrospinal fluid; Epigenetics; Locus coeruleus; Metals; Monoamines; Neurofibrillary degeneration; Non-cognitive symptoms; Nucleus raphe dorsalis; Phosphorylation; Sleep-wake cycle; Tau protein.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures






References
-
- Airaksinen MS, Paetau A, Paljarvi L, Reinikainen K, Riekkinen P, Suomalainen R, Panula P. Histamine neurons in human hypothalamus: anatomy in normal and Alzheimer diseased brains. Neuroscience. 1991;44:465–481. (PubMed: 1719449) - PubMed
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging – Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. (PubMed: 21514249) - PMC - PubMed
-
- Alzheimer´s Association. Alzheimer’s Association Trial Match. [Accessed 17 Apr 2014]; http://www.alz.org/research/clinical_trials/find_clinical_trials_trialma....
-
- Ambree O, Richter H, Sachser N, Lewejohann L, Dere E, de Souza Silva MA, Herring A, Keyvani K, Paulus W, Schabitz WR. Levodopa ameliorates learning and memory deficits in a murine model of Alzheimer’s disease. Neurobiol. Aging. 2009;30:1192–1204. (PubMed: 18079024) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

