The role of autophagy in cardiac hypertrophy
- PMID: 27084518
- PMCID: PMC4913516
- DOI: 10.1093/abbs/gmw025
The role of autophagy in cardiac hypertrophy
Abstract
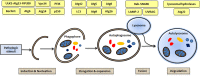
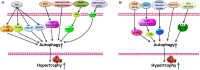
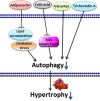
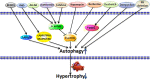
Autophagy is conserved in nature from lower eukaryotes to mammals and is an important self-cannibalizing, degradative process that contributes to the elimination of superfluous materials. Cardiac hypertrophy is primarily characterized by excess protein synthesis, increased cardiomyocyte size, and thickened ventricular walls and is a major risk factor that promotes arrhythmia and heart failure. In recent years, cardiomyocyte autophagy has been considered to play a role in controlling the hypertrophic response. However, the beneficial or aggravating role of cardiomyocyte autophagy in cardiac hypertrophy remains controversial. The exact mechanism of cardiomyocyte autophagy in cardiac hypertrophy requires further study. In this review, we summarize the controversies associated with autophagy in cardiac hypertrophy and provide insights into the role of autophagy in the development of cardiac hypertrophy. We conclude that future studies should emphasize the relationship between autophagy and the different stages of cardiac hypertrophy, as well as the autophagic flux and selective autophagy. Autophagy will be a potential therapeutic target for cardiac hypertrophy.
Keywords: autophagic flux; autophagy; cardiac function; cardiac hypertrophy; cell signaling.
© The Author 2016. Published by Oxford University Press on behalf of the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001, 104: 1615–1621. - PubMed
-
- Rothermel BA, Hill JA.. Myocyte autophagy in heart disease: friend or foe? Autophagy 2007, 3: 632–634. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

