Consensus Statement on Proton Therapy in Early-Stage and Locally Advanced Non-Small Cell Lung Cancer
- PMID: 27084663
- PMCID: PMC10868643
- DOI: 10.1016/j.ijrobp.2016.01.036
Consensus Statement on Proton Therapy in Early-Stage and Locally Advanced Non-Small Cell Lung Cancer
Abstract
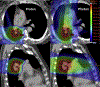
Radiation dose escalation has been shown to improve local control and survival in patients with non-small cell lung cancer in some studies, but randomized data have not supported this premise, possibly owing to adverse effects. Because of the physical characteristics of the Bragg peak, proton therapy (PT) delivers minimal exit dose distal to the target volume, resulting in better sparing of normal tissues in comparison to photon-based radiation therapy. This is particularly important for lung cancer given the proximity of the lung, heart, esophagus, major airways, large blood vessels, and spinal cord. However, PT is associated with more uncertainty because of the finite range of the proton beam and motion for thoracic cancers. PT is more costly than traditional photon therapy but may reduce side effects and toxicity-related hospitalization, which has its own associated cost. The cost of PT is decreasing over time because of reduced prices for the building, machine, maintenance, and overhead, as well as newer, shorter treatment programs. PT is improving rapidly as more research is performed particularly with the implementation of 4-dimensional computed tomography-based motion management and intensity modulated PT. Given these controversies, there is much debate in the oncology community about which patients with lung cancer benefit significantly from PT. The Particle Therapy Co-operative Group (PTCOG) Thoracic Subcommittee task group intends to address the issues of PT indications, advantages and limitations, cost-effectiveness, technology improvement, clinical trials, and future research directions. This consensus report can be used to guide clinical practice and indications for PT, insurance approval, and clinical or translational research directions.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: none.
Figures

References
-
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16:187–199. - PMC - PubMed
-
- Chun SG, Hu C, Choy H, et al. Outcomes of intensity modulated and 3D-conformal radiotherapy for stage III non-small cell lung cancer in NRG oncology/RTOG 0617 Presented at: 16th World Conference on Lung Cancer. September 6–10, 2015; Denver, CO.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

