Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy
- PMID: 27084741
- PMCID: PMC4843171
- DOI: 10.1158/1078-0432.CCR-15-1433
Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy
Abstract
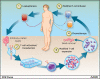
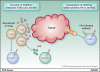
Chimeric antigen receptors (CAR) are engineered fusion proteins constructed from antigen recognition, signaling, and costimulatory domains that can be expressed in cytotoxic T cells with the purpose of reprograming the T cells to specifically target tumor cells. CAR T-cell therapy uses gene transfer technology to reprogram a patient's own T cells to stably express CARs, thereby combining the specificity of an antibody with the potent cytotoxic and memory functions of a T cell. In early-phase clinical trials, CAR T cells targeting CD19 have resulted in sustained complete responses within a population of otherwise refractory patients with B-cell malignancies and, more specifically, have shown complete response rates of approximately 90% in patients with relapsed or refractory acute lymphoblastic leukemia. Given this clinical efficacy, preclinical development of CAR T-cell therapy for a number of cancer indications has been actively investigated, and the future of the CAR T-cell field is extensive and dynamic. Several approaches to increase the feasibility and safety of CAR T cells are currently being explored, including investigation into the mechanisms regulating the persistence of CAR T cells. In addition, numerous early-phase clinical trials are now investigating CAR T-cell therapy beyond targeting CD19, especially in solid tumors. Trials investigating combinations of CAR T cells with immune checkpoint blockade therapies are now beginning and results are eagerly awaited. This review evaluates several of the ongoing and future directions of CAR T-cell therapy.
©2016 American Association for Cancer Research.
Figures

References
-
- Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161(6):2791–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

