Involvement of phosphorylated Apis mellifera CREB in gating a honeybee's behavioral response to an external stimulus
- PMID: 27084927
- PMCID: PMC4836635
- DOI: 10.1101/lm.040964.115
Involvement of phosphorylated Apis mellifera CREB in gating a honeybee's behavioral response to an external stimulus
Abstract
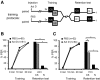
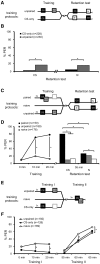
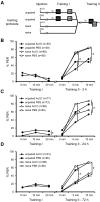
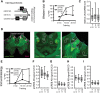
The transcription factor cAMP-response element-binding protein (CREB) is involved in neuronal plasticity. Phosphorylation activates CREB and an increased level of phosphorylated CREB is regarded as an indicator of CREB-dependent transcriptional activation. In honeybees(Apis mellifera)we recently demonstrated a particular high abundance of the phosphorylated honeybee CREB homolog (pAmCREB) in the central brain and in a subpopulation of mushroom body neurons. We hypothesize that these high pAmCREB levels are related to learning and memory formation. Here, we tested this hypothesis by analyzing brain pAmCREB levels in classically conditioned bees and bees experiencing unpaired presentations of conditioned stimulus (CS) and unconditioned stimulus (US). We demonstrate that both behavioral protocols display differences in memory formation but do not alter the level of pAmCREB in bee brains directly after training. Nevertheless, we report that bees responding to the CS during unpaired stimulus presentations exhibit higher levels of pAmCREB than nonresponding bees. In addition, Trichostatin A, a histone deacetylase inhibitor that is thought to enhance histone acetylation by CREB-binding protein, increases the bees' CS responsiveness. We conclude that pAmCREB is involved in gating a bee's behavioral response driven by an external stimulus.
© 2016 Gehring et al.; Published by Cold Spring Harbor Laboratory Press.
Figures

Similar articles
-
Abundance of phosphorylated Apis mellifera CREB in the honeybee's mushroom body inner compact cells varies with age.J Comp Neurol. 2016 Apr 15;524(6):1165-80. doi: 10.1002/cne.23894. Epub 2015 Sep 24. J Comp Neurol. 2016. PMID: 26355639
-
Duration of the unconditioned stimulus in appetitive conditioning of honeybees differentially impacts learning, long-term memory strength, and the underlying protein synthesis.Learn Mem. 2014 Nov 17;21(12):676-85. doi: 10.1101/lm.035600.114. Print 2014 Dec. Learn Mem. 2014. PMID: 25403456 Free PMC article.
-
Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation.J Neurosci. 2007 Jun 6;27(23):6128-40. doi: 10.1523/JNEUROSCI.0296-07.2007. J Neurosci. 2007. PMID: 17553985 Free PMC article.
-
Molecular mechanisms underlying formation of long-term reward memories and extinction memories in the honeybee (Apis mellifera).Learn Mem. 2014 Sep 15;21(10):534-42. doi: 10.1101/lm.033118.113. Print 2014 Oct. Learn Mem. 2014. PMID: 25225299 Free PMC article. Review.
-
[The morphological basis of conditioned reflex in the honeybee Apis mellifera L].Zh Vyssh Nerv Deiat Im I P Pavlova. 2012 Nov-Dec;62(6):654-63. Zh Vyssh Nerv Deiat Im I P Pavlova. 2012. PMID: 23530444 Review. Russian.
Cited by
-
Division of labor in honey bees is associated with transcriptional regulatory plasticity in the brain.J Exp Biol. 2019 Jul 16;222(Pt 14):jeb200196. doi: 10.1242/jeb.200196. J Exp Biol. 2019. PMID: 31138635 Free PMC article.
-
How can memories last for days, years, or a lifetime? Proposed mechanisms for maintaining synaptic potentiation and memory.Learn Mem. 2019 Apr 16;26(5):133-150. doi: 10.1101/lm.049395.119. Print 2019 May. Learn Mem. 2019. PMID: 30992383 Free PMC article. Review.
-
Context-dependent influence of threat on honey bee social network dynamics and brain gene expression.J Exp Biol. 2022 Mar 15;225(6):jeb243738. doi: 10.1242/jeb.243738. Epub 2022 Mar 28. J Exp Biol. 2022. PMID: 35202460 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
