The bile acid deoxycholate elicits defences in Arabidopsis and reduces bacterial infection
- PMID: 27085087
- PMCID: PMC6638291
- DOI: 10.1111/mpp.12416
The bile acid deoxycholate elicits defences in Arabidopsis and reduces bacterial infection
Abstract
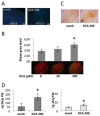
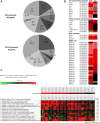
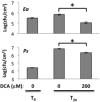
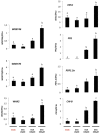
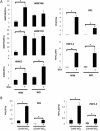
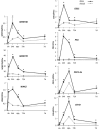
Disease has an effect on crop yields, causing significant losses. As the worldwide demand for agricultural products increases, there is a need to pursue the development of new methods to protect crops from disease. One mechanism of plant protection is through the activation of the plant immune system. By exogenous application, 'plant activator molecules' with elicitor properties can be used to activate the plant immune system. These defence-inducing molecules represent a powerful and often environmentally friendly tool to fight pathogens. We show that the secondary bile acid deoxycholic acid (DCA) induces defence in Arabidopsis and reduces the proliferation of two bacterial phytopathogens: Erwinia amylovora and Pseudomonas syringae pv. tomato. We describe the global defence response triggered by this new plant activator in Arabidopsis at the transcriptional level. Several induced genes were selected for further analysis by quantitative reverse transcription-polymerase chain reaction. We describe the kinetics of their induction and show that abiotic stress, such as moderate drought or nitrogen limitation, does not impede DCA induction of defence. Finally, we investigate the role in the activation of defence by this bile acid of the salicylic acid biosynthesis gene SID2, of the receptor-like kinase family genes WAK1-3 and of the NADPH oxidase-encoding RbohD gene. Altogether, we show that DCA constitutes a promising molecule for plant protection which can induce complementary lines of defence, such as callose deposition, reactive oxygen species accumulation and the jasmonic acid and salicylic acid signalling pathways.
Keywords: Erwinia amylovora; Pseudomonas syringae; SID2; WAK; bile acid; elicitor; plant defence.
© 2016 BSPP AND JOHN WILEY & SONS LTD.
Figures

Similar articles
-
Chitosan Oligosaccharide Induces Resistance to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis thaliana by Activating Both Salicylic Acid- and Jasmonic Acid-Mediated Pathways.Mol Plant Microbe Interact. 2018 Dec;31(12):1271-1279. doi: 10.1094/MPMI-03-18-0071-R. Epub 2018 Oct 4. Mol Plant Microbe Interact. 2018. PMID: 29869942
-
Holaphyllamine, a steroid, is able to induce defense responses in Arabidopsis thaliana and increases resistance against bacterial infection.Planta. 2017 Dec;246(6):1109-1124. doi: 10.1007/s00425-017-2755-z. Epub 2017 Aug 16. Planta. 2017. PMID: 28815300
-
Bacillus cereus AR156 Activates Defense Responses to Pseudomonas syringae pv. tomato in Arabidopsis thaliana Similarly to flg22.Mol Plant Microbe Interact. 2018 Mar;31(3):311-322. doi: 10.1094/MPMI-10-17-0240-R. Epub 2018 Jan 8. Mol Plant Microbe Interact. 2018. PMID: 29090631
-
Singlet oxygen-mediated and EXECUTER-dependent signalling and acclimation of Arabidopsis thaliana exposed to light stress.Philos Trans R Soc Lond B Biol Sci. 2014 Mar 3;369(1640):20130227. doi: 10.1098/rstb.2013.0227. Print 2014 Apr 19. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24591714 Free PMC article. Review.
-
Immune priming in plants: from the onset to transgenerational maintenance.Essays Biochem. 2022 Sep 30;66(5):635-646. doi: 10.1042/EBC20210082. Essays Biochem. 2022. PMID: 35822618 Free PMC article. Review.
Cited by
-
LPMO-oxidized cellulose oligosaccharides evoke immunity in Arabidopsis conferring resistance towards necrotrophic fungus B. cinerea.Commun Biol. 2021 Jun 11;4(1):727. doi: 10.1038/s42003-021-02226-7. Commun Biol. 2021. PMID: 34117349 Free PMC article.
-
Foliar Application or Seed Priming of Cholic Acid-Glycine Conjugates can Mitigate/Prevent the Rice Bacterial Leaf Blight Disease via Activating Plant Defense Genes.Front Plant Sci. 2021 Sep 24;12:746912. doi: 10.3389/fpls.2021.746912. eCollection 2021. Front Plant Sci. 2021. PMID: 34630495 Free PMC article.
-
Biotransformation of Waste Bile Acids: A New Possible Sustainable Approach to Anti-Fungal Molecules for Crop Plant Bioprotection?Int J Mol Sci. 2022 Apr 8;23(8):4152. doi: 10.3390/ijms23084152. Int J Mol Sci. 2022. PMID: 35456970 Free PMC article.
-
A Major Facilitator Superfamily Peptide Transporter From Fusarium oxysporum Influences Bioethanol Production From Lignocellulosic Material.Front Microbiol. 2019 Feb 26;10:295. doi: 10.3389/fmicb.2019.00295. eCollection 2019. Front Microbiol. 2019. PMID: 30863378 Free PMC article.
-
Plant nitrogen supply affects the Botrytis cinerea infection process and modulates known and novel virulence factors.Mol Plant Pathol. 2020 Nov;21(11):1436-1450. doi: 10.1111/mpp.12984. Epub 2020 Sep 17. Mol Plant Pathol. 2020. PMID: 32939948 Free PMC article.
References
-
- Crow, J.P. (1997) Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide, 1, 145–157. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

