Crystal structure of carbonmonoxy sickle hemoglobin in R-state conformation
- PMID: 27085422
- PMCID: PMC4859812
- DOI: 10.1016/j.jsb.2016.04.003
Crystal structure of carbonmonoxy sickle hemoglobin in R-state conformation
Abstract
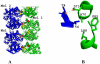
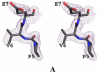
The fundamental pathophysiology of sickle cell disease is predicated by the polymerization of deoxygenated (T-state) sickle hemoglobin (Hb S) into fibers that distort red blood cells into the characteristic sickle shape. The crystal structure of deoxygenated Hb S (DeoxyHb S) and other studies suggest that the polymer is initiated by a primary interaction between the mutation βVal6 from one Hb S molecule, and a hydrophobic acceptor pocket formed by the residues βAla70, βPhe85 and βLeu88 of an adjacent located Hb S molecule. On the contrary, oxygenated or liganded Hb S does not polymerize or incorporate in the polymer. In this paper we present the crystal structure of carbonmonoxy-ligated sickle Hb (COHb S) in the quaternary classical R-state at 1.76Å. The overall structure and the pathological donor and acceptor environments of COHb S are similar to those of the isomorphous CO-ligated R-state normal Hb (COHb A), but differ significantly from DeoxyHb S as expected. More importantly, the packing of COHb S molecules does not show the typical pathological interaction between βVal6 and the βAla70, βPhe85 and βLeu88 hydrophobic acceptor pocket observed in DeoxyHb S crystal. The structural analysis of COHb S, COHb A and DeoxyHb S provides atomic level insight into why liganded hemoglobin does not form a polymer.
Keywords: Allosteric; Crystal structure; Hemoglobin; Mutation; R-state; Sickle cell disease.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Adachi K, Kim J, Kinney TR, Asakura T. Effect of the beta 73 amino acid on the hydrophobicity, solubility, and the kinetics of polymerization of deoxyhemoglobin S. The Journal of Biological Chemistry. 1987;262:10470–10474. - PubMed
-
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Cryst. 2010;D66:213–221. - PMC - PubMed
-
- Akinsheye I, Klings ES. Sickle cell anemia and vascular dysfunction: The nitric oxide connection. Journal of Cellular Physiology. 2010;224:620–625. - PubMed
-
- Baldwin J, Chothia C. Haemoglobin: The structural changes related to ligand binding and its allosteric mechanism. Journal of Molecular Biology. 1979;129:175–220. - PubMed
-
- Benesch RE, Yung S, Benesch R, Mack J, Schneider RG. Alpha-chain contacts in the polymerisation of sickle haemogloblin. Nature. 1976;260:219–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

