The mutation spectrum in genomic late replication domains shapes mammalian GC content
- PMID: 27085808
- PMCID: PMC4872117
- DOI: 10.1093/nar/gkw268
The mutation spectrum in genomic late replication domains shapes mammalian GC content
Abstract
Genome sequence compositions and epigenetic organizations are correlated extensively across multiple length scales. Replication dynamics, in particular, is highly correlated with GC content. We combine genome-wide time of replication (ToR) data, topological domains maps and detailed functional epigenetic annotations to study the correlations between replication timing and GC content at multiple scales. We find that the decrease in genomic GC content at large scale late replicating regions can be explained by mutation bias favoring A/T nucleotide, without selection or biased gene conversion. Quantification of the free dNTP pool during the cell cycle is consistent with a mechanism involving replication-coupled mutation spectrum that favors AT nucleotides at late S-phase. We suggest that mammalian GC content composition is shaped by independent forces, globally modulating mutation bias and locally selecting on functional element. Deconvoluting these forces and analyzing them on their native scales is important for proper characterization of complex genomic correlations.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

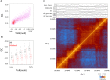

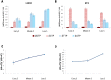
References
-
- Woodfine K., Fiegler H., Beare D.M., Collins J.E., McCann O.T., Young B.D., Debernardi S., Mott R., Dunham I., Carter N.P. Replication timing of the human genome. Hum. Mol. Genet. 2004;13:191–202. - PubMed
-
- Bernardi G., Olofsson B., Filipski J., Zerial M., Salinas J., Cuny G., Meunier-Rotival M., Rodier F. The mosaic genome of warm-blooded vertebrates. Science. 1985;228:953–958. - PubMed
-
- Wolfe K.H., Sharp P.M., Li W.H. Mutation rates differ among regions of the mammalian genome. Nature. 1989;337:283–285. - PubMed
-
- Arndt P.F., Hwa T., Petrov D.A. Substantial regional variation in substitution rates in the human genome: importance of GC content, gene density, and telomere-specific effects. J. Mol. Evol. 2005;60:748–763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

