Meningococcal Vaccinations
- PMID: 27086142
- PMCID: PMC4929086
- DOI: 10.1007/s40121-016-0107-0
Meningococcal Vaccinations
Abstract
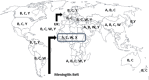
Neisseria meningitidis, a gram-negative diplococcal bacterium, is a common asymptomatic nasopharyngeal colonizer that may infrequently lead to invasive disease in the form of meningitis or bacteremia. Six serogroups (A, B, C, W, X and Y) are responsible for the majority of invasive infections. Increased risk of disease occurs in specific population groups including infants, adolescents, those with asplenia or complement deficiencies, and those residing in crowded living conditions such as in college dormitories. The incidence of invasive meningococcal disease varies geographically with some countries (e.g., in the African meningitis belt) having both high endemic disease rates and ongoing epidemics, with annual rates reaching 1000 cases per 100,000 persons. Given the significant morbidity and mortality associated with meningococcal disease, it remains a major global health threat best prevented by vaccination. Several countries have implemented vaccination programs with the selection of specific vaccine(s) based on locally prevalent serogroup(s) of N. meningitidis and targeting population groups at highest risk. Polysaccharide meningococcal vaccines became available over 40 years ago, but are limited by their inability to produce immunologic memory responses, poor immunogenicity in infants/children, hyporesponsiveness after repeated doses, and lack of efficacy against nasopharyngeal carriage. In 1999, the first meningococcal conjugate vaccines were introduced and have been successful in overcoming many of the shortcomings of polysaccharide vaccines. The implementation of meningococcal conjugate vaccination programs in many areas of the world (including the massive campaign in sub-Saharan Africa using a serogroup A conjugate vaccine) has led to dramatic reductions in the incidence of meningococcal disease by both individual and population protection. Progressive advances in vaccinology have led to the recent licensure of two effective vaccines against serogroup B [MenB-4C (Bexsero) and MenB-FHbp (Trumenba)]. Overall, the evolution of novel meningococcal vaccines and the effective implementation of targeted vaccination programs has led to a substantial decrease in the burden of disease worldwide representing a major public health accomplishment.
Keywords: Meningococcal; Neisseria meningitidis; Review; Vaccinations.
Figures
References
-
- Stephens DS, Apicella MA. Neisseria meningitidis. In: Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 8th ed. Philadelphia, PA Elsevier Saunders Inc.; 2015. p. 2425-45.
-
- Marchiafava E, Celli A. Spra i micrococchi della meningite cerebrospinale epidemica. Gazz degli Ospedali. 1884;5:59.
-
- Weichselbaum A. Ueber die aetiologie der akuten meningitis cerebrospinalis. Fortschr Med. 1887;5:573.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical